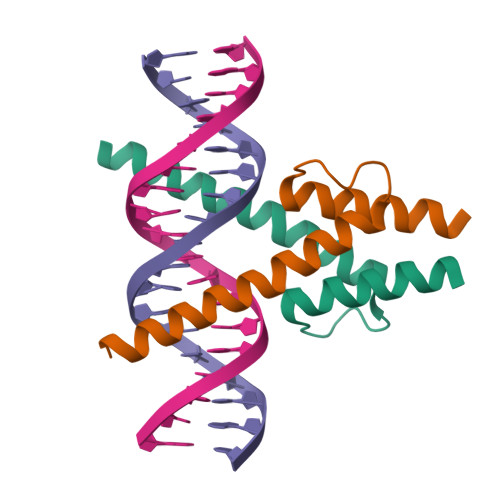
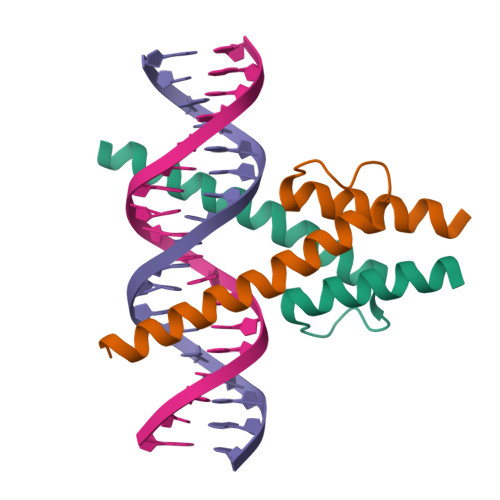
Interfacial water confers transcription factors with dinucleotide specificity.
Morgunova, E., Nagy, G., Yin, Y., Zhu, F., Nayak, S.P., Xiao, T., Sokolov, I., Popov, A., Laughton, C., Grubmuller, H., Taipale, J.(2025) Nat Struct Mol Biol
- PubMed: 39753777
- DOI: https://doi.org/10.1038/s41594-024-01449-6
- Primary Citation of Related Structures:
7Z5I, 7Z5K, 8PM5, 8PM7, 8PMC, 8PMF, 8PMN, 8PMV, 8PN4, 8PNA, 8PNC - PubMed Abstract:
Transcription factors (TFs) recognize specific bases within their DNA-binding motifs, with each base contributing nearly independently to total binding energy. However, the energetic contributions of particular dinucleotides can deviate strongly from the additive approximation, indicating that some TFs can specifically recognize DNA dinucleotides. Here we solved high-resolution (<1 Å) structures of MYF5 and BARHL2 bound to DNAs containing sets of dinucleotides that have different affinities to the proteins. The dinucleotides were recognized either enthalpically, by an extensive water network that connects the adjacent bases to the TF, or entropically, by a hydrophobic patch that maintained interfacial water mobility. This mechanism confers differential temperature sensitivity to the optimal sites, with implications for thermal regulation of gene expression. Our results uncover the enigma of how TFs can recognize more complex local features than mononucleotides and demonstrate that water-mediated recognition is important for predicting affinities of macromolecules from their sequence.
Organizational Affiliation:
Department of Medical Biochemistry and Biophysics, Karolinska Institutet, Stockholm, Sweden.