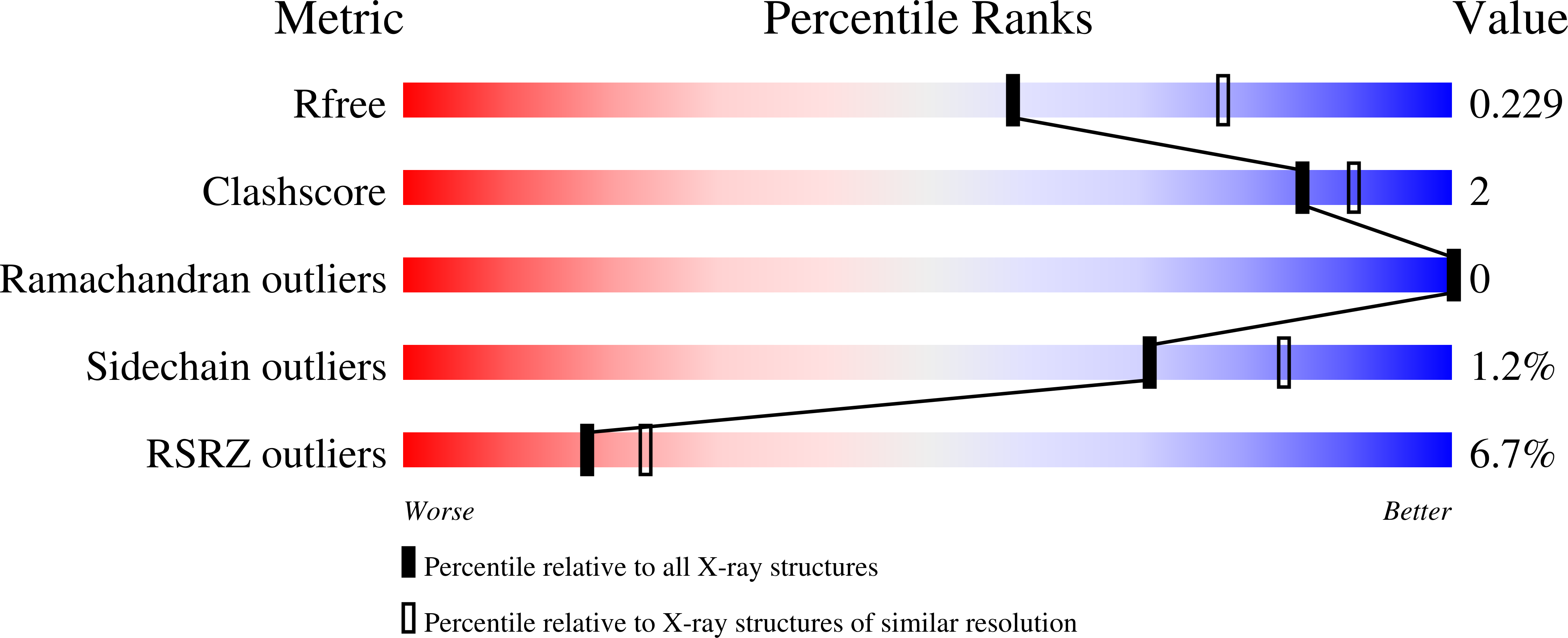
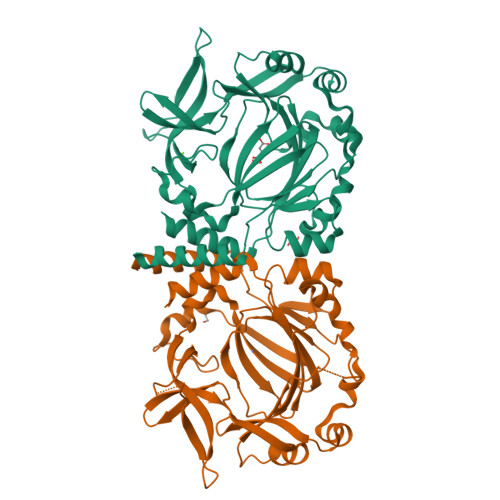
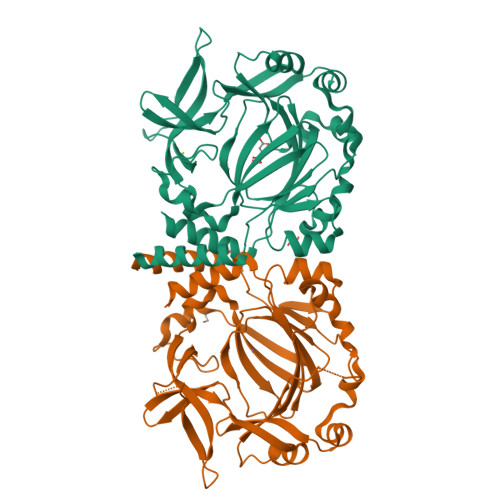
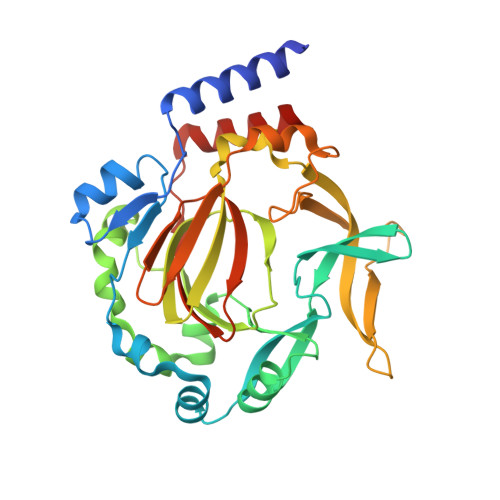
Conservation of the unusual dimeric JmjC fold of JMJD7 from Drosophila melanogaster to humans.
Chowdhury, R., Abboud, M.I., Wiley, J., Tumber, A., Markolovic, S., Schofield, C.J.(2022) Sci Rep 12: 6065-6065
- PubMed: 35410347
- DOI: https://doi.org/10.1038/s41598-022-10028-y
- Primary Citation of Related Structures:
7YXG, 7YXH, 7YXI, 7YXJ, 7YXK, 7YXL - PubMed Abstract:
The JmjC family of 2-oxoglutarate dependent oxygenases catalyse a range of hydroxylation and demethylation reactions in humans and other animals. Jumonji domain-containing 7 (JMJD7) is a JmjC (3S)-lysyl-hydroxylase that catalyses the modification of Developmentally Regulated GTP Binding Proteins 1 and 2 (DRG1 and 2); JMJD7 has also been reported to have histone endopeptidase activity. Here we report biophysical and biochemical studies on JMJD7 from Drosophila melanogaster (dmJMJD7). Notably, crystallographic analyses reveal that the unusual dimerization mode of JMJD7, which involves interactions between both the N- and C-terminal regions of both dmJMJD7 monomers and disulfide formation, is conserved in human JMJD7 (hsJMJD7). The results further support the assignment of JMJD7 as a lysyl hydroxylase and will help enable the development of selective inhibitors for it and other JmjC oxygenases.
Organizational Affiliation:
Chemistry Research Laboratory, Department of Chemistry and the Ineos Oxford Institute for Antimicrobial Research, University of Oxford, Mansfield Road, Oxford, OX1 3TA, UK.