Immunoglobulin M perception by Fc mu R.
Li, Y., Shen, H., Zhang, R., Ji, C., Wang, Y., Su, C., Xiao, J.(2023) Nature 615: 907-912
- PubMed: 36949194
- DOI: https://doi.org/10.1038/s41586-023-05835-w
- Primary Citation of Related Structures:
7YSG, 7YTC, 7YTD, 7YTE - PubMed Abstract:
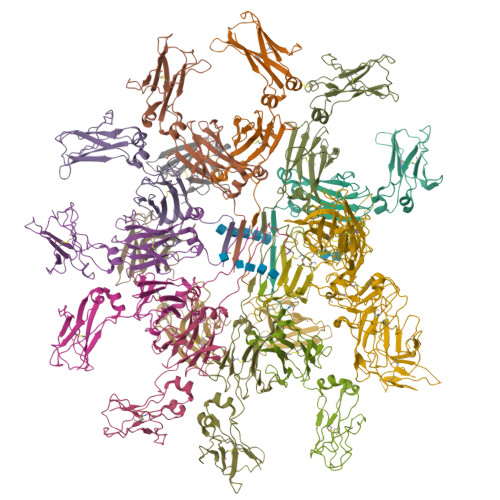
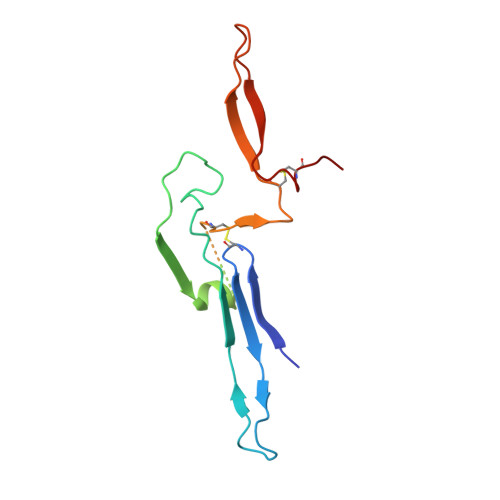
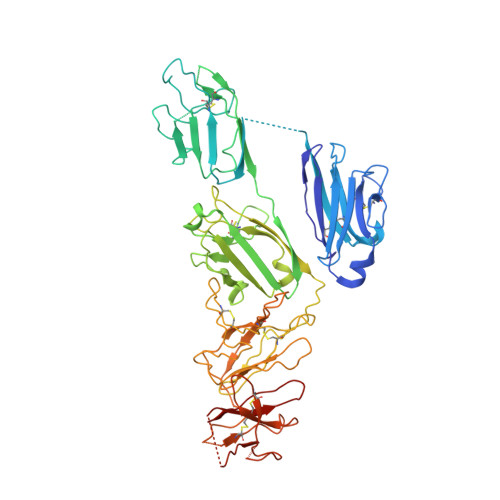
Immunoglobulin M (IgM) is the first antibody to emerge during embryonic development and the humoral immune response 1 . IgM can exist in several distinct forms, including monomeric, membrane-bound IgM within the B cell receptor (BCR) complex, pentameric and hexameric IgM in serum and secretory IgM on the mucosal surface. FcμR, the only IgM-specific receptor in mammals, recognizes different forms of IgM to regulate diverse immune responses 2-5 . However, the underlying molecular mechanisms remain unknown. Here we delineate the structural basis of the FcμR-IgM interaction by crystallography and cryo-electron microscopy. We show that two FcμR molecules interact with a Fcμ-Cμ4 dimer, suggesting that FcμR can bind to membrane-bound IgM with a 2:1 stoichiometry. Further analyses reveal that FcμR-binding sites are accessible in the context of IgM BCR. By contrast, pentameric IgM can recruit four FcμR molecules to bind on the same side and thereby facilitate the formation of an FcμR oligomer. One of these FcμR molecules occupies the binding site of the secretory component. Nevertheless, four FcμR molecules bind to the other side of secretory component-containing secretory IgM, consistent with the function of FcμR in the retrotransport of secretory IgM. These results reveal intricate mechanisms of IgM perception by FcμR.
Organizational Affiliation:
State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking University, Beijing, P. R. China.