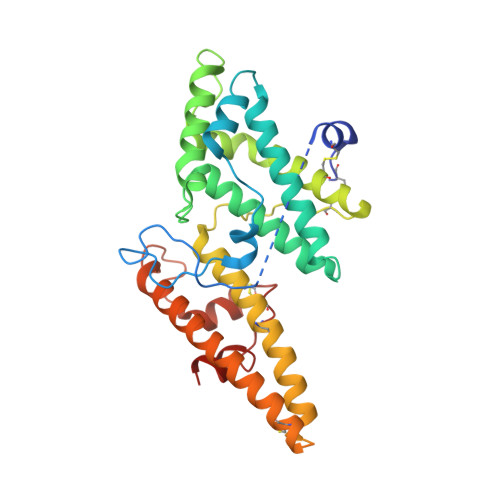
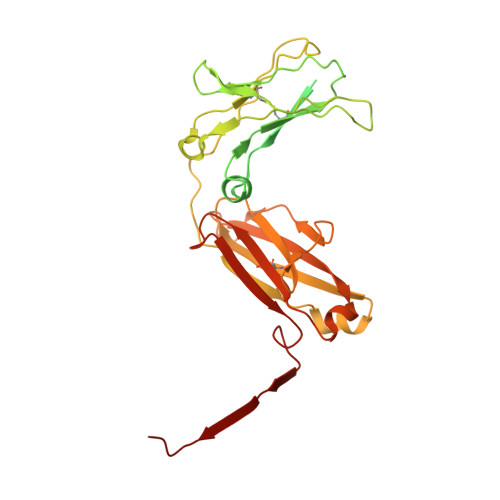
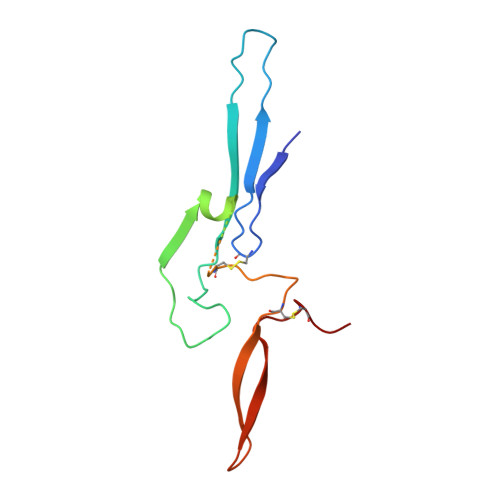
Plasmodium falciparum has evolved multiple mechanisms to hijack human immunoglobulin M.
Ji, C., Shen, H., Su, C., Li, Y., Chen, S., Sharp, T.H., Xiao, J.(2023) Nat Commun 14: 2650-2650
- PubMed: 37156765
- DOI: https://doi.org/10.1038/s41467-023-38320-z
- Primary Citation of Related Structures:
7Y09, 7Y0H, 7Y0J, 7YG2 - PubMed Abstract:
Plasmodium falciparum causes the most severe malaria in humans. Immunoglobulin M (IgM) serves as the first line of humoral defense against infection and potently activates the complement pathway to facilitate P. falciparum clearance. A number of P. falciparum proteins bind IgM, leading to immune evasion and severe disease. However, the underlying molecular mechanisms remain unknown. Here, using high-resolution cryo-electron microscopy, we delineate how P. falciparum proteins VAR2CSA, TM284VAR1, DBLMSP, and DBLMSP2 target IgM. Each protein binds IgM in a different manner, and together they present a variety of Duffy-binding-like domain-IgM interaction modes. We further show that these proteins interfere directly with IgM-mediated complement activation in vitro, with VAR2CSA exhibiting the most potent inhibitory effect. These results underscore the importance of IgM for human adaptation of P. falciparum and provide critical insights into its immune evasion mechanism.
Organizational Affiliation:
State Key Laboratory of Protein and Plant Gene Research, School of Life Sciences, Peking University, Beijing, China.