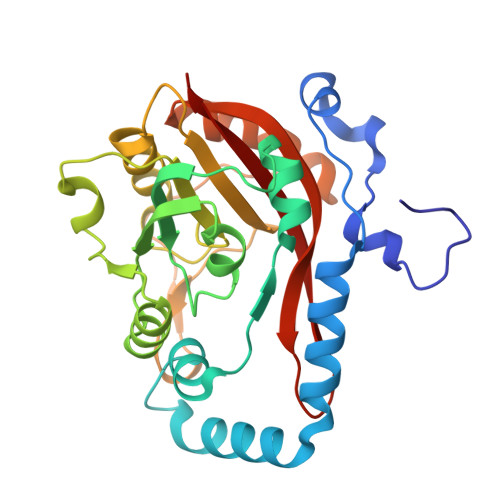
Molecular basis for METTL9-mediated N1-histidine methylation.
Wang, X., Xie, H., Guo, Q., Cao, D., Ru, W., Zhao, S., Zhu, Z., Zhang, J., Pan, W., Yao, X., Xu, C.(2023) Cell Discov 9: 38-38
- PubMed: 37015930
- DOI: https://doi.org/10.1038/s41421-023-00548-w
- Primary Citation of Related Structures:
7Y9C, 7YF2, 7YF3, 7YF4
Organizational Affiliation:
MOE Key Laboratory for Membraneless Organelles and Cellular Dynamics, Hefei National Research Center for Interdisciplinary Sciences at the Microscale, Division of Life Sciences and Medicine, University of Science and Technology of China, Hefei, China.