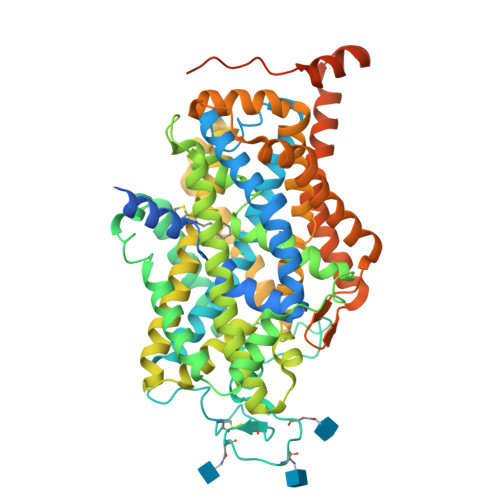
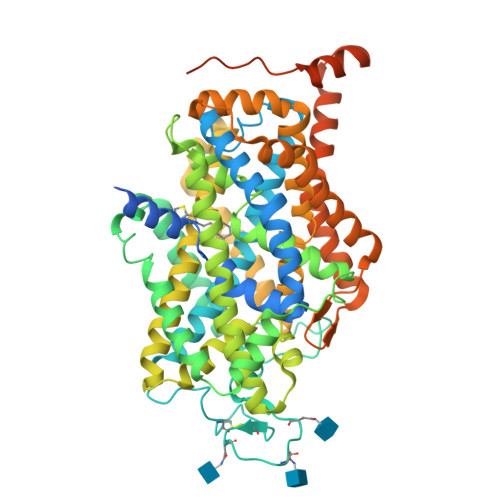
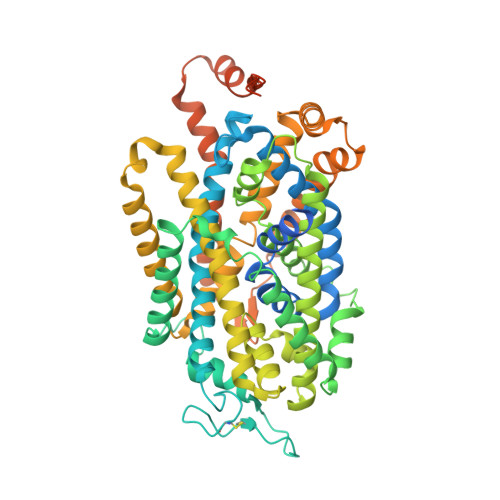
Molecular basis for substrate recognition and transport of human GABA transporter GAT1.
Zhu, A., Huang, J., Kong, F., Tan, J., Lei, J., Yuan, Y., Yan, C.(2023) Nat Struct Mol Biol 30: 1012-1022
- PubMed: 37400655
- DOI: https://doi.org/10.1038/s41594-023-00983-z
- Primary Citation of Related Structures:
7Y7V, 7Y7W, 7Y7Y, 7Y7Z - PubMed Abstract:
γ-Aminobutyric acid (GABA), an important inhibitory neurotransmitter in the central nervous system, is recycled through specific GABA transporters (GATs). GAT1, which is mainly expressed in the presynaptic terminals of axons, is a potential drug target of neurological disorders due to its essential role in GABA transport. Here we report four cryogenic electron microscopy structures of human GAT1, at resolutions of 2.2-3.2 Å. GAT1 in substrate-free form or in complex with the antiepileptic drug tiagabine exhibits an inward-open conformation. In the presence of GABA or nipecotic acid, inward-occluded structures are captured. The GABA-bound structure reveals an interaction network bridged by hydrogen bonds and ion coordination for GABA recognition. The substrate-free structure unwinds the last helical turn of transmembrane helix TM1a to release sodium ions and substrate. Complemented by structure-guided biochemical analyses, our studies reveal detailed mechanism of GABA recognition and transport, and elucidate mode of action of the inhibitors, nipecotic acid and tiagabine.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, Beijing Frontier Research Center for Biological Structure, Beijing Advanced Innovation Center for Structural Biology, Tsinghua-Peking Joint Center for Life Sciences, School of Life Sciences, Tsinghua University, Beijing, China.