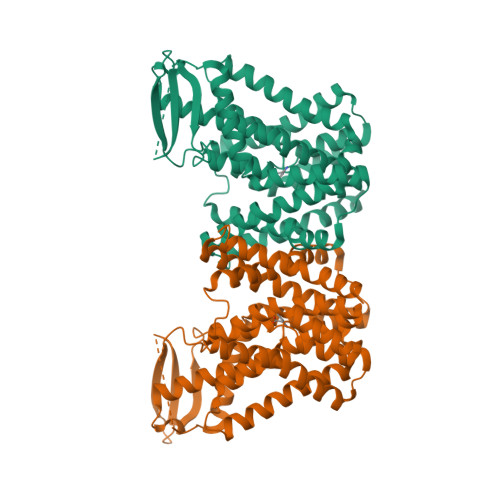
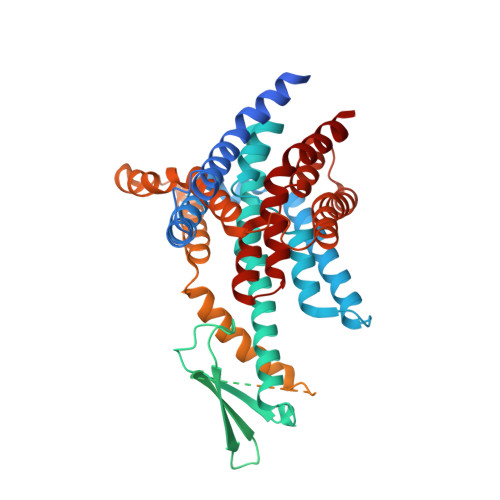
Structures and mechanisms of the Arabidopsis auxin transporter PIN3.
Su, N., Zhu, A., Tao, X., Ding, Z.J., Chang, S., Ye, F., Zhang, Y., Zhao, C., Chen, Q., Wang, J., Zhou, C.Y., Guo, Y., Jiao, S., Zhang, S., Wen, H., Ma, L., Ye, S., Zheng, S.J., Yang, F., Wu, S., Guo, J.(2022) Nature 609: 616-621
- PubMed: 35917926
- DOI: https://doi.org/10.1038/s41586-022-05142-w
- Primary Citation of Related Structures:
7WKS, 7WKW, 7XXB - PubMed Abstract:
The PIN-FORMED (PIN) protein family of auxin transporters mediates polar auxin transport and has crucial roles in plant growth and development 1,2 . Here we present cryo-electron microscopy structures of PIN3 from Arabidopsis thaliana in the apo state and in complex with its substrate indole-3-acetic acid and the inhibitor N-1-naphthylphthalamic acid (NPA). A. thaliana PIN3 exists as a homodimer, and its transmembrane helices 1, 2 and 7 in the scaffold domain are involved in dimerization. The dimeric PIN3 forms a large, joint extracellular-facing cavity at the dimer interface while each subunit adopts an inward-facing conformation. The structural and functional analyses, along with computational studies, reveal the structural basis for the recognition of indole-3-acetic acid and NPA and elucidate the molecular mechanism of NPA inhibition on PIN-mediated auxin transport. The PIN3 structures support an elevator-like model for the transport of auxin, whereby the transport domains undergo up-down rigid-body motions and the dimerized scaffold domains remain static.
Organizational Affiliation:
Department of Biophysics and Department of Neurology of the Fourth Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China.