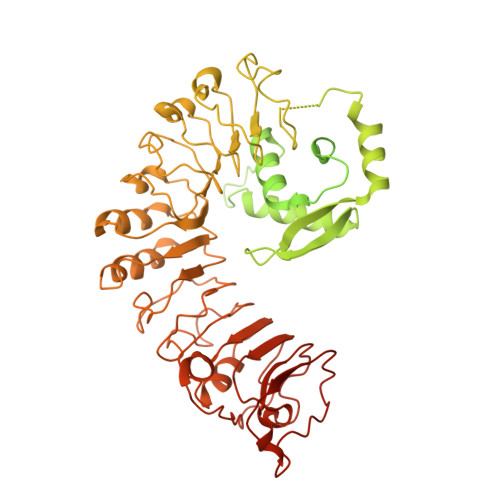
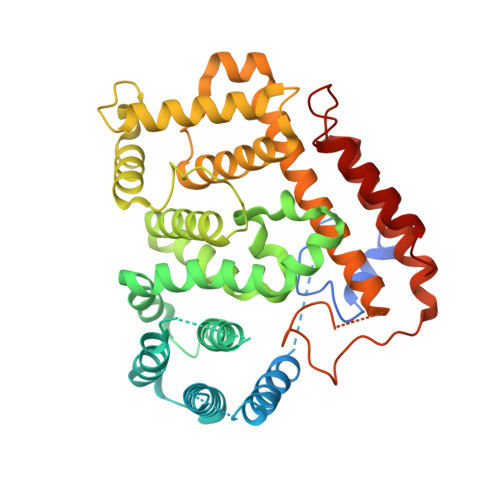
Pathogen effector AvrSr35 triggers Sr35 resistosome assembly via a direct recognition mechanism.
Zhao, Y.B., Liu, M.X., Chen, T.T., Ma, X., Li, Z.K., Zheng, Z., Zheng, S.R., Chen, L., Li, Y.Z., Tang, L.R., Chen, Q., Wang, P., Ouyang, S.(2022) Sci Adv 8: eabq5108-eabq5108
- PubMed: 36083908
- DOI: https://doi.org/10.1126/sciadv.abq5108
- Primary Citation of Related Structures:
7XDS, 7XE0, 7XVG, 7XX2 - PubMed Abstract:
Nucleotide-binding, leucine-rich repeat receptors (NLRs) perceive pathogen effectors to trigger plant immunity. The direct recognition mechanism of pathogen effectors by coiled-coil NLRs (CNLs) remains unclear. We demonstrate that the Triticum monococcum CNL Sr35 directly recognizes the pathogen effector AvrSr35 from Puccinia graminis f. sp . tritici and report a cryo-electron microscopy structure of Sr35 resistosome and a crystal structure of AvrSr35. We show that AvrSr35 forms homodimers that are disassociated into monomers upon direct recognition by the leucine-rich repeat domain of Sr35, which induces Sr35 resistosome assembly and the subsequent immune response. The first 20 amino-terminal residues of Sr35 are indispensable for immune signaling but not for plasma membrane association. Our findings reveal the direct recognition and activation mechanism of a plant CNL and provide insights into biochemical function of Sr35 resistosome.
Organizational Affiliation:
Provincial University Key Laboratory of Cellular Stress Response and Metabolic Regulation, the Key Laboratory of Innate Immune Biology of Fujian Province, Biomedical Research Center of South China, Key Laboratory of OptoElectronic Science and Technology for Medicine of the Ministry of Education, College of Life Sciences, Fujian Normal University, Fuzhou 350117, China.