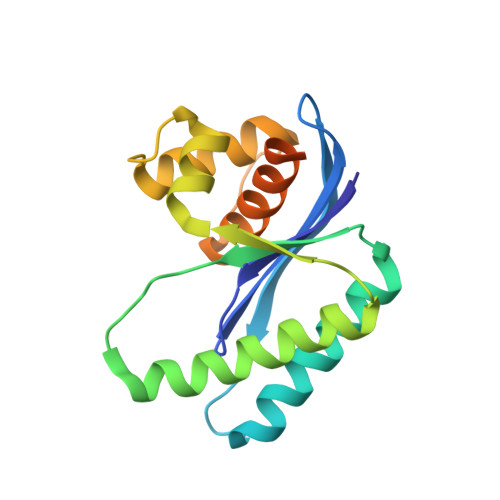
Structural and Functional Characterization of the Holliday Junction Resolvase RuvC from Deinococcus radiodurans.
Qin, C., Han, W., Xu, Y., Zhao, Y., Xu, H., Tian, B., Wang, L., Hua, Y.(2022) Microorganisms 10
- PubMed: 35744678
- DOI: https://doi.org/10.3390/microorganisms10061160
- Primary Citation of Related Structures:
7XHJ - PubMed Abstract:
Holliday junctions (HJs) are four-way DNA structures, which are an important intermediate in the process of homologous recombination. In most bacteria, HJs are cleaved by specific nucleases called RuvC resolvases at the end of homologous recombination. Deinococcus radiodurans is an extraordinary radiation-resistant bacterium and is known as an ideal model organism for elucidating DNA repair processes. Here, we described the biochemical properties and the crystal structure of RuvC from D. radiodurans ( Dr RuvC). Dr RuvC exhibited an RNase H fold that belonged to the retroviral integrase family. Among many DNA substrates, Dr RuvC specifically bound to HJ DNA and cleaved it. In particular, Mn 2+ was the preferred bivalent metal co-factor for HJ cleavage, whereas high concentrations of Mg 2+ inhibited the binding of Dr RuvC to HJ. In addition, Dr RuvC was crystallized and the crystals diffracted to 1.6 Å. The crystal structure of Dr RuvC revealed essential amino acid sites for cleavage and binding activities, indicating that Dr RuvC was a typical resolvase with a characteristic choice for metal co-factor.
Organizational Affiliation:
MOE Key Laboratory of Biosystems Homeostasis and Protection, Institute of Biophysics, College of Life Sciences, Zhejiang University, Hangzhou 310058, China.