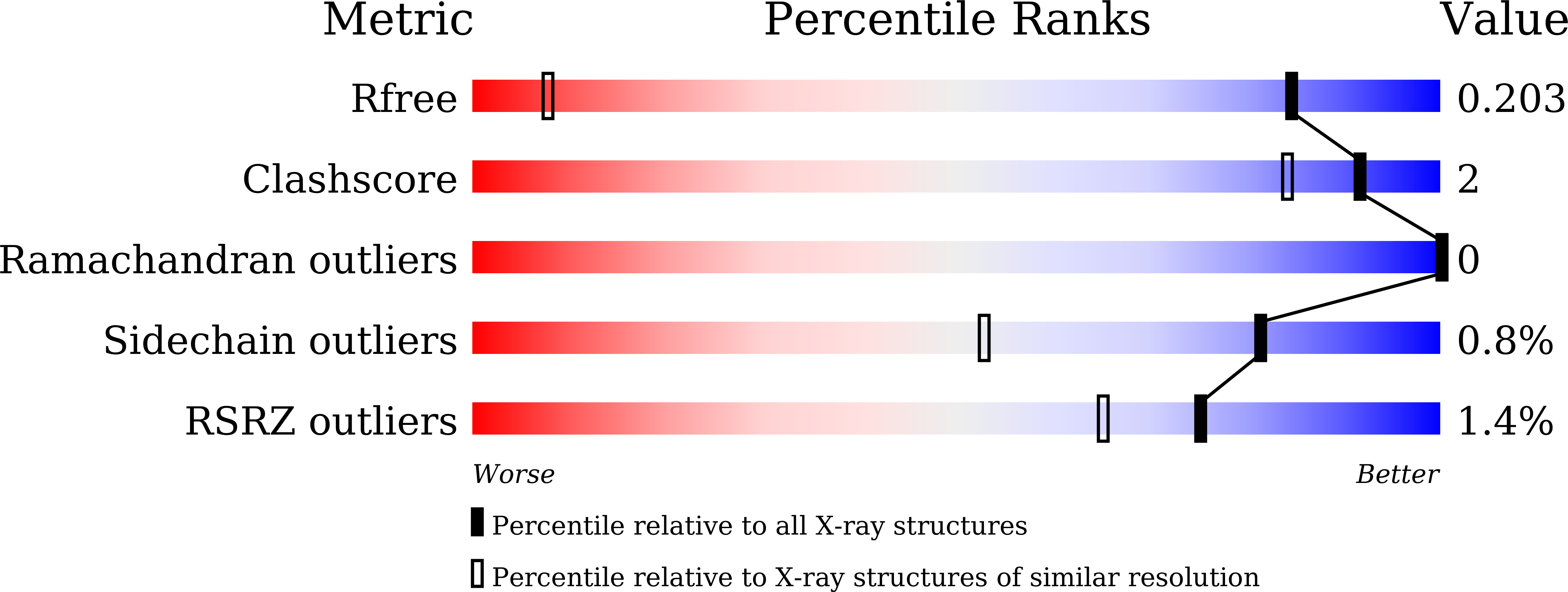
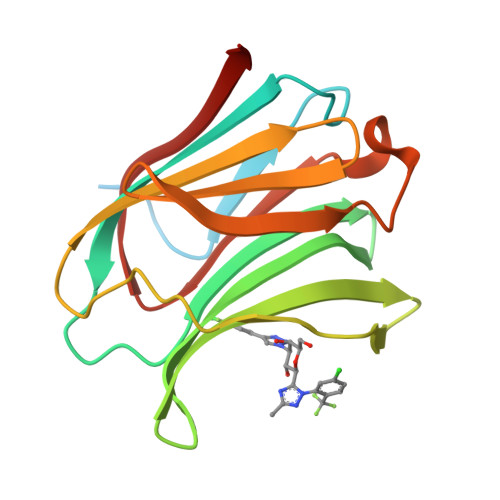
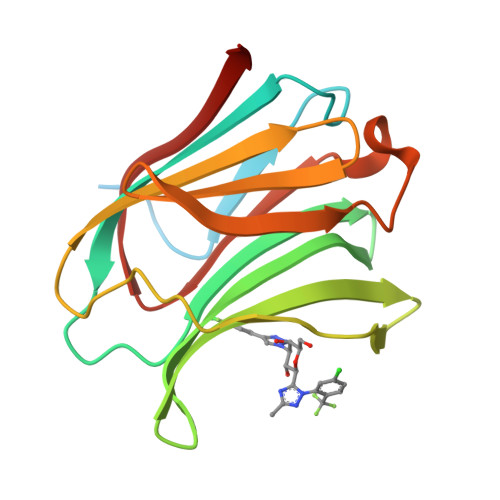
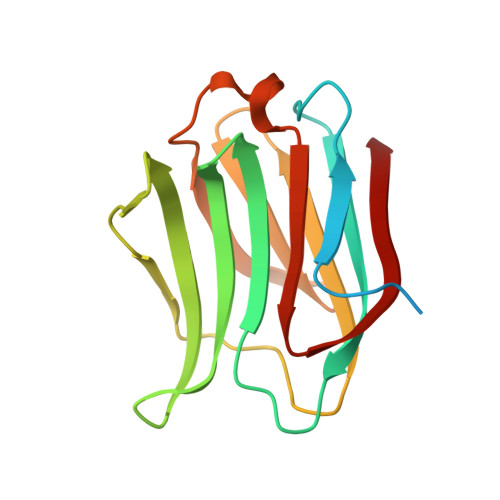
Identification of Monosaccharide Derivatives as Potent, Selective, and Orally Bioavailable Inhibitors of Human and Mouse Galectin-3.
Liu, C., Jalagam, P.R., Feng, J., Wang, W., Raja, T., Sura, M.R., Manepalli, R.K.V.L.P., Aliphedi, B.R., Medavarapu, S., Nair, S.K., Muthalagu, V., Natesan, R., Gupta, A., Beno, B., Panda, M., Ghosh, K., Shukla, J.K., Sale, H., Haldar, P., Kalidindi, N., Shah, D., Patel, D., Mathur, A., Ellsworth, B.A., Cheng, D., Regueiro-Ren, A.(2022) J Med Chem 65: 11084-11099
- PubMed: 35969688
- DOI: https://doi.org/10.1021/acs.jmedchem.2c00517
- Primary Citation of Related Structures:
7XFA - PubMed Abstract:
Galectin-3 (Gal-3), a member of the β-galactoside-binding protein family, is implicated in a wide variety of human diseases. Identification of Gal-3 inhibitors with the right combination of potency (against both human and mouse Gal-3) and pharmacokinetic properties to fully evaluate the potential of Gal-3 for therapeutic intervention has been a major challenge due to the characteristics of its binding pocket: high hydrophilicity and key structural differences between human Gal-3 and the mouse ortholog. We report the discovery of a novel series of monosaccharide-based, highly potent, and orally bioavailable inhibitors of human and mouse Gal-3. The novel monosaccharide derivatives proved to be selective for Gal-3, the only member of the chimeric type of galectins, over Gal-1 and Gal-9, representative of the prototype and tandem-repeat type of galectins, respectively. The proposed binding mode for the newly identified ligands was confirmed by an X-ray cocrystal structure of a representative analogue bound to Gal-3 protein.
Organizational Affiliation:
Department of Small Molecule Drug Discovery, Research & Early Development, Bristol Myers Squibb Company, Princeton, New Jersey 08543, United States.