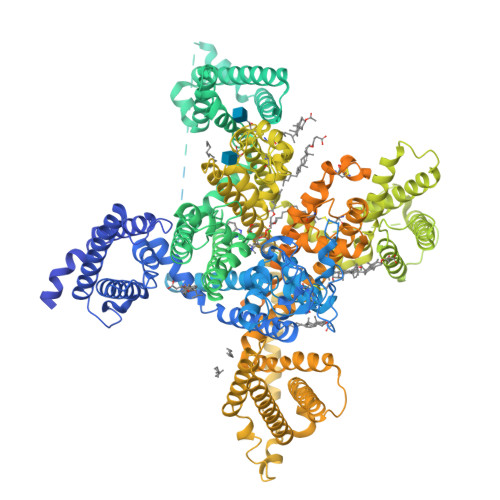
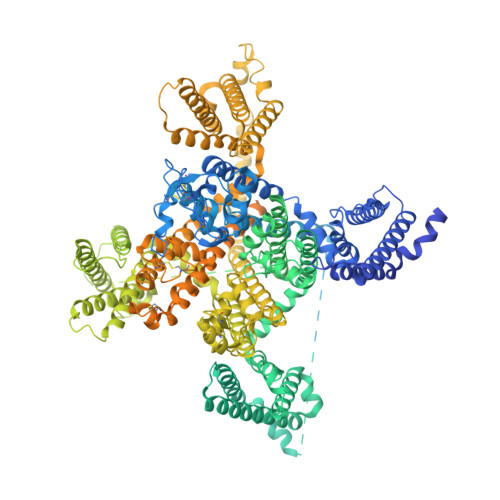
Structure, gating, and pharmacology of human Ca V 3.3 channel.
He, L., Yu, Z., Geng, Z., Huang, Z., Zhang, C., Dong, Y., Gao, Y., Wang, Y., Chen, Q., Sun, L., Ma, X., Huang, B., Wang, X., Zhao, Y.(2022) Nat Commun 13: 2084-2084
- PubMed: 35440630
- DOI: https://doi.org/10.1038/s41467-022-29728-0
- Primary Citation of Related Structures:
7WLI, 7WLJ, 7WLK, 7WLL - PubMed Abstract:
The low-voltage activated T-type calcium channels regulate cellular excitability and oscillatory behavior of resting membrane potential which trigger many physiological events and have been implicated with many diseases. Here, we determine structures of the human T-type Ca V 3.3 channel, in the absence and presence of antihypertensive drug mibefradil, antispasmodic drug otilonium bromide and antipsychotic drug pimozide. Ca V 3.3 contains a long bended S6 helix from domain III, with a positive charged region protruding into the cytosol, which is critical for T-type Ca V channel activation at low voltage. The drug-bound structures clearly illustrate how these structurally different compounds bind to the same central cavity inside the Ca V 3.3 channel, but are mediated by significantly distinct interactions between drugs and their surrounding residues. Phospholipid molecules penetrate into the central cavity in various extent to shape the binding pocket and play important roles in stabilizing the inhibitor. These structures elucidate mechanisms of channel gating, drug recognition, and actions, thus pointing the way to developing potent and subtype-specific drug for therapeutic treatments of related disorders.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.