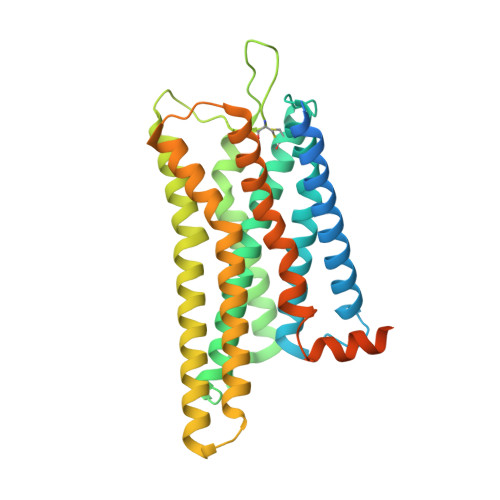
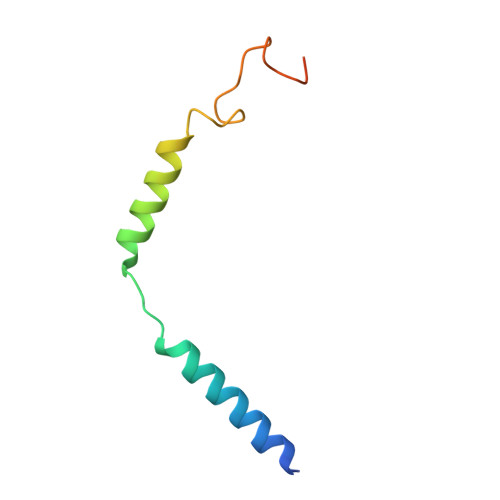
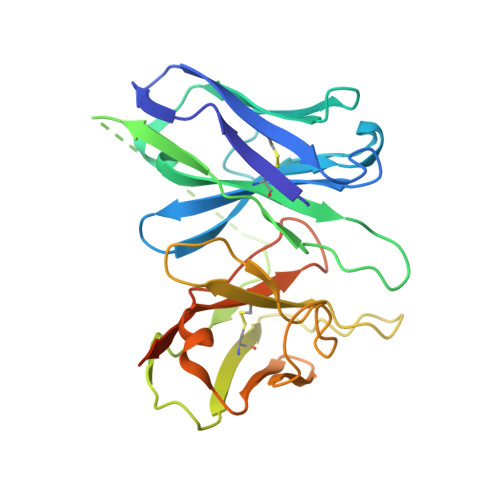
Structures of the endogenous peptide- and selective non-peptide agonist-bound SSTR2 signaling complexes.
Chen, L.N., Wang, W.W., Dong, Y.J., Shen, D.D., Guo, J., Yu, X., Qin, J., Ji, S.Y., Zhang, H., Shen, Q., He, Q., Yang, B., Zhang, Y., Li, Q., Mao, C.(2022) Cell Res 32: 785-788
- PubMed: 35578016
- DOI: https://doi.org/10.1038/s41422-022-00669-z
- Primary Citation of Related Structures:
7WIC, 7WIG
Organizational Affiliation:
Department of Biophysics and Department of Pathology of Sir Run Run Shaw Hospital, Zhejiang University, School of Medicine, Hangzhou, Zhejiang, China.