Crystal Structure of the Oxomolybdenum Mesoporphyrin IX-Reconstituted CYP102A1 (P450BM3) Heme Domain with N-Hexadecanoyl-L-Homoserine
Karasawa, M., Stanfield, J.K., Kasai, C., Sugimoto, H., Shoji, O.To be published.
Experimental Data Snapshot
Starting Model: experimental
View more details
Entity ID: 1 | |||||
|---|---|---|---|---|---|
| Molecule | Chains | Sequence Length | Organism | Details | Image |
| Bifunctional cytochrome P450/NADPH--P450 reductase | 456 | Priestia megaterium | Mutation(s): 0 Gene Names: cyp102A1 EC: 1.14.14.1 (PDB Primary Data), 1.6.2.4 (PDB Primary Data) | 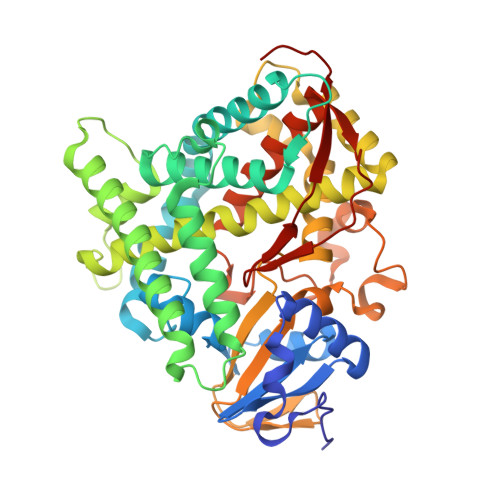 | |
UniProt | |||||
Find proteins for P14779 (Priestia megaterium (strain ATCC 14581 / DSM 32 / CCUG 1817 / JCM 2506 / NBRC 15308 / NCIMB 9376 / NCTC 10342 / NRRL B-14308 / VKM B-512 / Ford 19)) Explore P14779 Go to UniProtKB: P14779 | |||||
Entity Groups | |||||
| Sequence Clusters | 30% Identity50% Identity70% Identity90% Identity95% Identity100% Identity | ||||
| UniProt Group | P14779 | ||||
Sequence AnnotationsExpand | |||||
| |||||
| Ligands 3 Unique | |||||
|---|---|---|---|---|---|
| ID | Chains | Name / Formula / InChI Key | 2D Diagram | 3D Interactions | |
| MI9 (Subject of Investigation/LOI) Query on MI9 | C [auth A], G [auth B] | Oxomolybdenum Mesoporphyrin IX C34 H36 Mo N4 O5 BVPDTXBFNWLJAZ-HXFTUNQESA-L |  | ||
| 8PD (Subject of Investigation/LOI) Query on 8PD | D [auth A], H [auth B] | (2~{S})-2-(hexadecanoylamino)-4-oxidanyl-butanoic acid C20 H39 N O4 GYSOJFKWYUVNCE-SFHVURJKSA-N |  | ||
| GOL Query on GOL | E [auth A], F [auth A], I [auth B], J [auth B] | GLYCEROL C3 H8 O3 PEDCQBHIVMGVHV-UHFFFAOYSA-N |  | ||
| Length ( Å ) | Angle ( ˚ ) |
|---|---|
| a = 58.754 | α = 90 |
| b = 146.716 | β = 98.04 |
| c = 62.854 | γ = 90 |
| Software Name | Purpose |
|---|---|
| REFMAC | refinement |
| XDS | data reduction |
| XSCALE | data scaling |
| MOLREP | phasing |
| PDB_EXTRACT | data extraction |
| Funding Organization | Location | Grant Number |
|---|---|---|
| Japan Society for the Promotion of Science (JSPS) | Japan | JP15H05806 |
| Japan Society for the Promotion of Science (JSPS) | Japan | JP19J23669 |
| Japan Society for the Promotion of Science (JSPS) | Japan | JP21H04704 |
| Japan Science and Technology | Japan | JPMJCR15P3 |