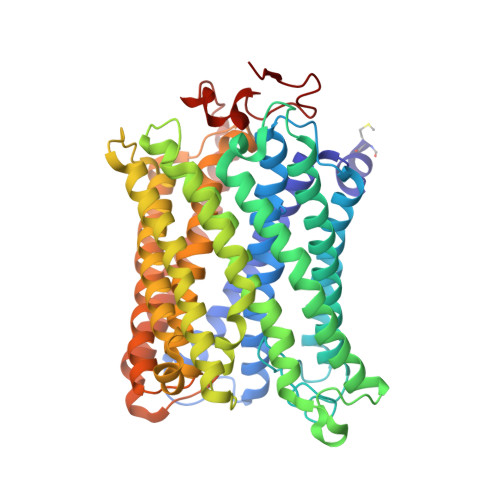
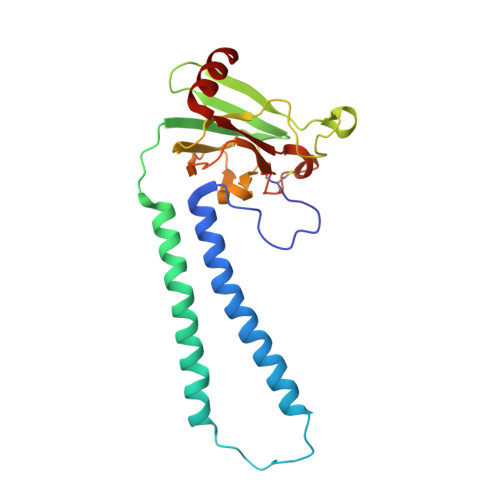
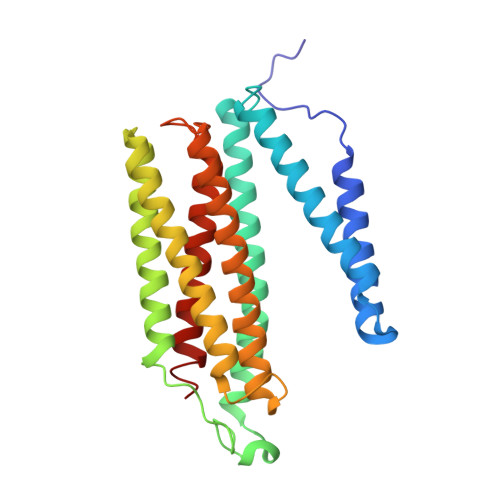
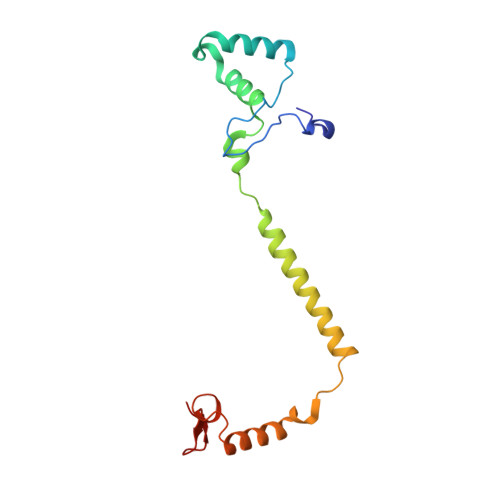
Crystallographic cyanide-probing for cytochrome c oxidase reveals structural bases suggesting that a putative proton transfer H-pathway pumps protons.
Shimada, A., Baba, J., Nagao, S., Shinzawa-Itoh, K., Yamashita, E., Muramoto, K., Tsukihara, T., Yoshikawa, S.(2023) J Biol Chem 299: 105277-105277
- PubMed: 37742916
- DOI: https://doi.org/10.1016/j.jbc.2023.105277
- Primary Citation of Related Structures:
7VUW, 7VVR, 7W3E - PubMed Abstract:
Cytochrome c oxidase (CcO) reduces O 2 in the O 2 -reduction site by sequential four-electron donations through the low-potential metal sites (Cu A and Fe a ). Redox-coupled X-ray crystal structural changes have been identified at five distinct sites including Asp 51 , Arg 438 , Glu 198 , the hydroxyfarnesyl ethyl group of heme a, and Ser 382 , respectively. These sites interact with the putative proton-pumping H-pathway. However, the metal sites responsible for each structural change have not been identified, since these changes were detected as structural differences between the fully reduced and fully oxidized CcOs. Thus, the roles of these structural changes in the CcO function are yet to be revealed. X-ray crystal structures of cyanide-bound CcOs under various oxidation states showed that the O 2 -reduction site controlled only the Ser 382 -including site, while the low-potential metal sites induced the other changes. This finding indicates that these low-potential site-inducible structural changes are triggered by sequential electron-extraction from the low-potential sites by the O 2 -reduction site and that each structural change is insensitive to the oxidation and ligand-binding states of the O 2 -reduction site. Because the proton/electron coupling efficiency is constant (1:1), regardless of the reaction progress in the O 2 -reduction site, the structural changes induced by the low-potential sites are assignable to those critically involved in the proton pumping, suggesting that the H-pathway, facilitating these low-potential site-inducible structural changes, pumps protons. Furthermore, a cyanide-bound CcO structure suggests that a hypoxia-inducible activator, Higd1a, activates the O 2 -reduction site without influencing the electron transfer mechanism through the low-potential sites, kinetically confirming that the low-potential sites facilitate proton pump.
Organizational Affiliation:
Picobiology Institute, Graduate School of Life Science, University of Hyogo, Hyogo, Japan.