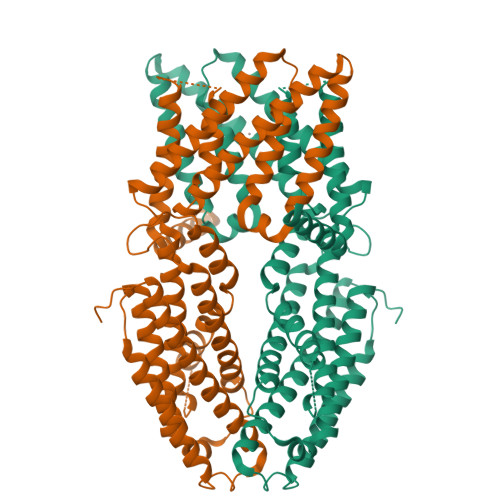
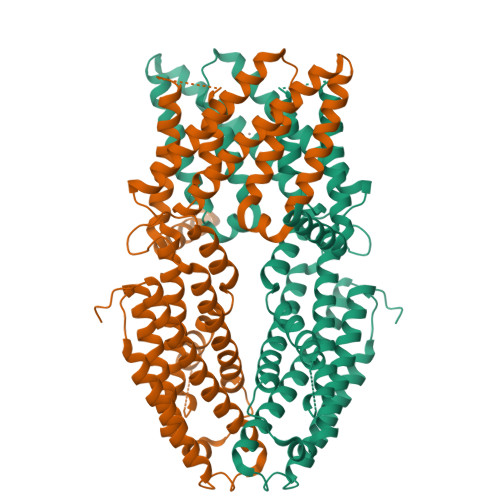
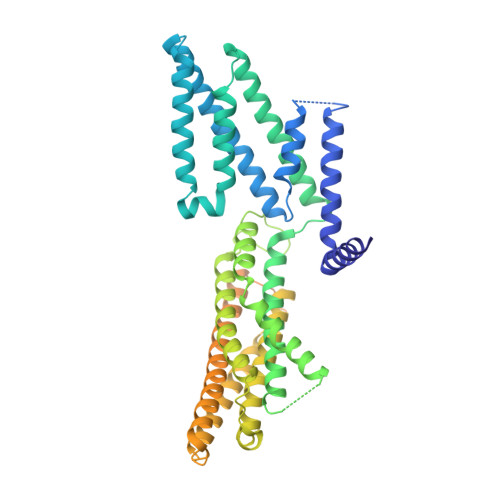
Structural basis of ALMT1-mediated aluminum resistance in Arabidopsis.
Wang, J., Yu, X., Ding, Z.J., Zhang, X., Luo, Y., Xu, X., Xie, Y., Li, X., Yuan, T., Zheng, S.J., Yang, W., Guo, J.(2022) Cell Res 32: 89-98
- PubMed: 34799726
- DOI: https://doi.org/10.1038/s41422-021-00587-6
- Primary Citation of Related Structures:
7VOJ, 7VQ3, 7VQ4, 7VQ5, 7VQ7 - PubMed Abstract:
The plant aluminum (Al)-activated malate transporter ALMT1 mediates the efflux of malate to chelate the Al in acidic soils and underlies the plant Al resistance. Here we present cryo-electron microscopy (cryo-EM) structures of Arabidopsis thaliana ALMT1 (AtALMT1) in the apo, malate-bound, and Al-bound states at neutral and/or acidic pH at up to 3.0 Å resolution. The AtALMT1 dimer assembles an anion channel and each subunit contains six transmembrane helices (TMs) and six cytosolic α-helices. Two pairs of Arg residues are located in the center of the channel pore and contribute to malate recognition. Al binds at the extracellular side of AtALMT1 and induces conformational changes of the TM1-2 loop and the TM5-6 loop, resulting in the opening of the extracellular gate. These structures, along with electrophysiological measurements, molecular dynamic simulations, and mutagenesis study in Arabidopsis, elucidate the structural basis for Al-activated malate transport by ALMT1.
Organizational Affiliation:
Department of Biophysics, and Department of Pathology of Sir Run Run Shaw Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, China.