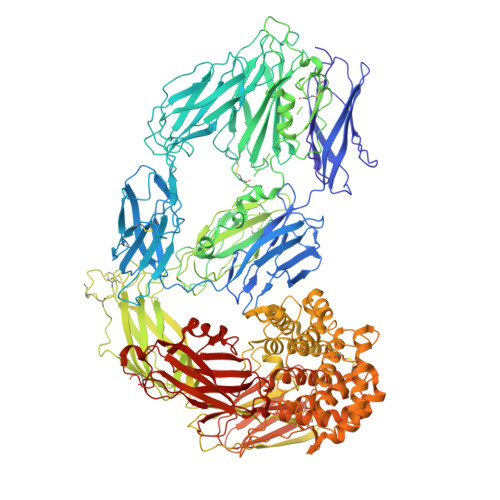
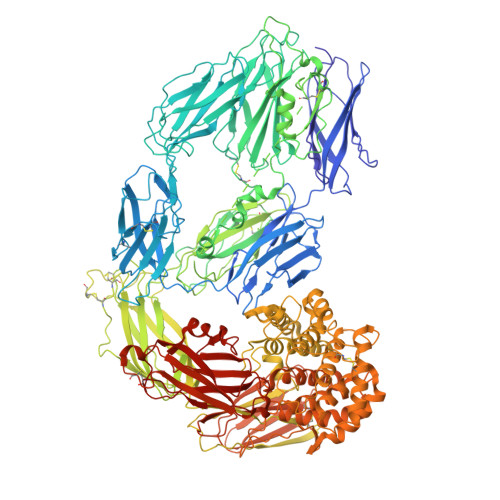
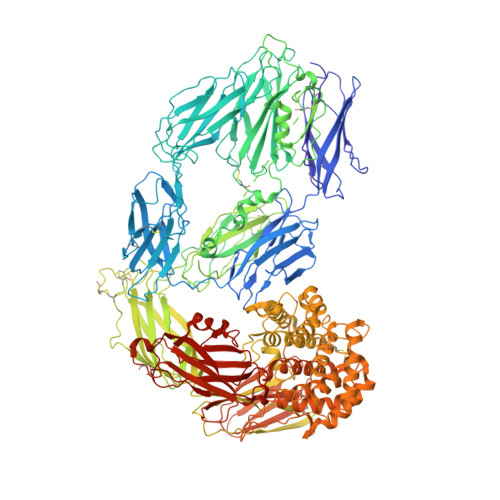
Cryo-EM structures reveal the dynamic transformation of human alpha-2-macroglobulin working as a protease inhibitor.
Huang, X., Wang, Y., Yu, C., Zhang, H., Ru, Q., Li, X., Song, K., Zhou, M., Zhu, P.(2022) Sci China Life Sci 65: 2491-2504
- PubMed: 35781771
- DOI: https://doi.org/10.1007/s11427-022-2139-2
- Primary Citation of Related Structures:
7VON, 7VOO - PubMed Abstract:
Human alpha-2-macroglobulin is a well-known inhibitor of a broad spectrum of proteases and plays important roles in immunity, inflammation, and infections. Here, we report the cryo-EM structures of human alpha-2-macroglobulin in its native state, induced state transformed by its authentic substrate, human trypsin, and serial intermediate states between the native and fully induced states. These structures exhibit distinct conformations, which reveal the dynamic transformation of alpha-2-macro-globulin that acts as a protease inhibitor. The results shed light on the molecular mechanism of alpha-2-macroglobulin in entrapping substrates.
Organizational Affiliation:
National Laboratory of Biomacromolecules, CAS Center for Excellence in Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, Beijing, 100101, China.