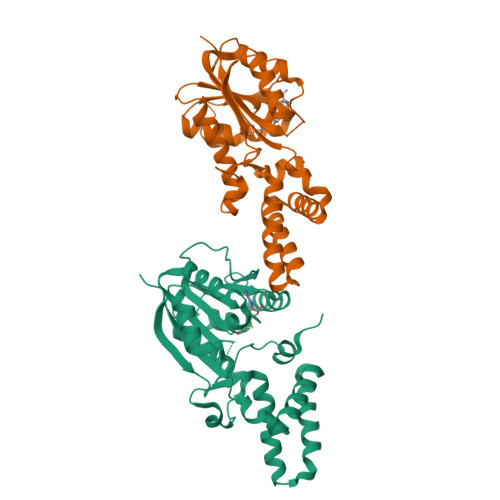
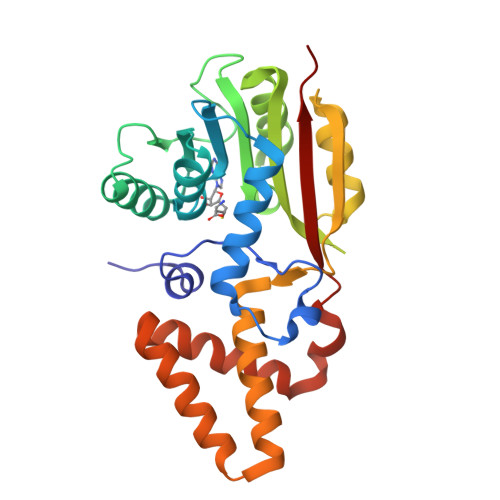
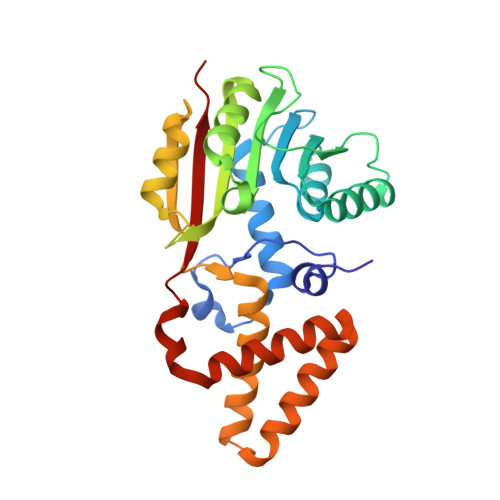
Evidence for an Enzyme-Catalyzed Rauhut-Currier Reaction during the Biosynthesis of Spinosyn A.
Choi, S.H., Jeon, B., Kim, N., Wu, H.H., Ko, T.P., Ruszczycky, M.W., Isiorho, E.A., Liu, Y.N., Keatinge-Clay, A.T., Tsai, M.D., Liu, H.W.(2021) J Am Chem Soc 143: 20291-20295
- PubMed: 34813308
- DOI: https://doi.org/10.1021/jacs.1c09482
- Primary Citation of Related Structures:
7V6H - PubMed Abstract:
The catalog of enzymes known to catalyze the nucleophile-assisted formation of C-C bonds is extremely small, and there is presently no definitive example of a biological Rauhut-Currier reaction. Biosynthesis of the polyketide insecticide spinosyn A in Saccharopolyspora spinosa involves a [4 + 2]-cycloaddition and a subsequent intramolecular C-C bond formation catalyzed by SpnF and SpnL, respectively. Isotope tracer experiments and kinetic isotope effects, however, imply that the SpnL-catalyzed reaction proceeds without initial deprotonation of the substrate. The crystal structure of SpnL exhibits high similarity to SAM-dependent methyltransferases as well as SpnF. The residue Cys60 is also shown to reside in the SpnL active site, and the Cys60Ala SpnL mutant is found to be devoid of activity. Moreover, SpnL is covalently modified at Cys60 and irreversibly inactivated when it is coincubated with a fluorinated substrate analogue designed as a suicide inactivator of nucleophile-assisted C-C bond formation. These results suggest that SpnL catalyzes a biological Rauhut-Currier reaction.
Organizational Affiliation:
Department of Chemistry, University of Texas at Austin, Austin, Texas 78712, United States.