Structural basis for mechanotransduction in a potassium-dependent mechanosensitive ion channel.
Mount, J., Maksaev, G., Summers, B.T., Fitzpatrick, J.A.J., Yuan, P.(2022) Nat Commun 13: 6904-6904
- PubMed: 36371466
- DOI: https://doi.org/10.1038/s41467-022-34737-0
- Primary Citation of Related Structures:
7UW5, 7UX1 - PubMed Abstract:
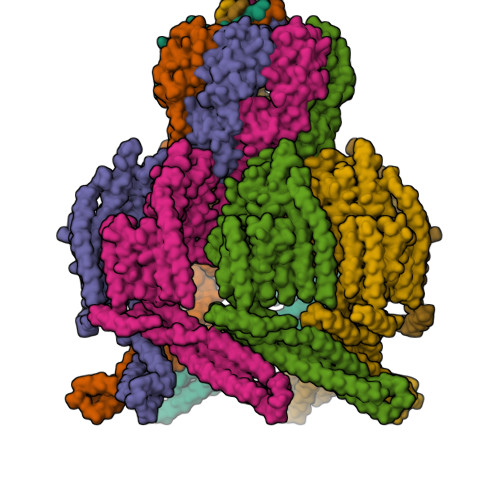
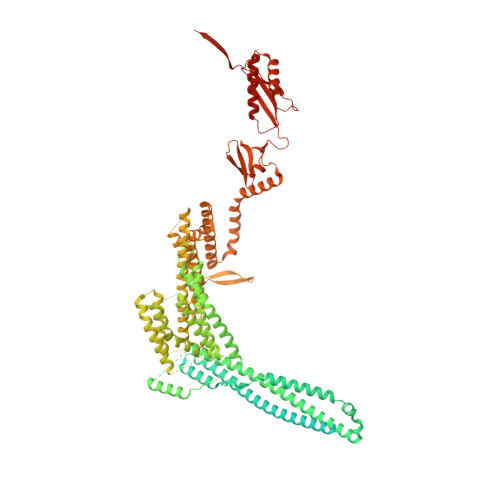
Mechanosensitive channels of small conductance, found in many living organisms, open under elevated membrane tension and thus play crucial roles in biological response to mechanical stress. Amongst these channels, MscK is unique in that its activation also requires external potassium ions. To better understand this dual gating mechanism by force and ligand, we elucidate distinct structures of MscK along the gating cycle using cryo-electron microscopy. The heptameric channel comprises three layers: a cytoplasmic domain, a periplasmic gating ring, and a markedly curved transmembrane domain that flattens and expands upon channel opening, which is accompanied by dilation of the periplasmic ring. Furthermore, our results support a potentially unifying mechanotransduction mechanism in ion channels depicted as flattening and expansion of the transmembrane domain.
Organizational Affiliation:
Department of Cell Biology and Physiology, Washington University School of Medicine, Saint Louis, MO, USA.