Allosteric regulation of glycogen breakdown by the second messenger cyclic di-GMP.
Schumacher, M.A., Wormann, M.E., Henderson, M., Salinas, R., Latoscha, A., Al-Bassam, M.M., Singh, K.S., Barclay, E., Gunka, K., Tschowri, N.(2022) Nat Commun 13: 5834-5834
- PubMed: 36192422
- DOI: https://doi.org/10.1038/s41467-022-33537-w
- Primary Citation of Related Structures:
7U39, 7U3A, 7U3B, 7U3D - PubMed Abstract:
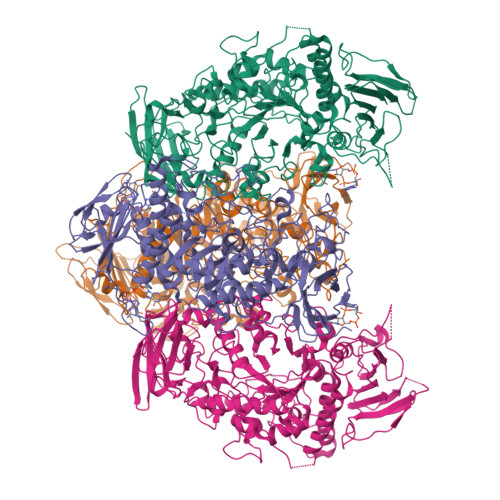
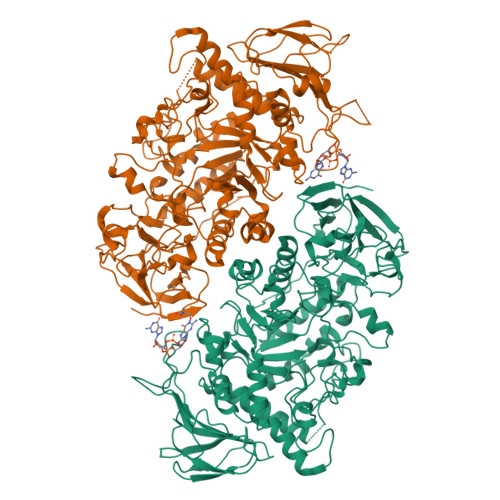
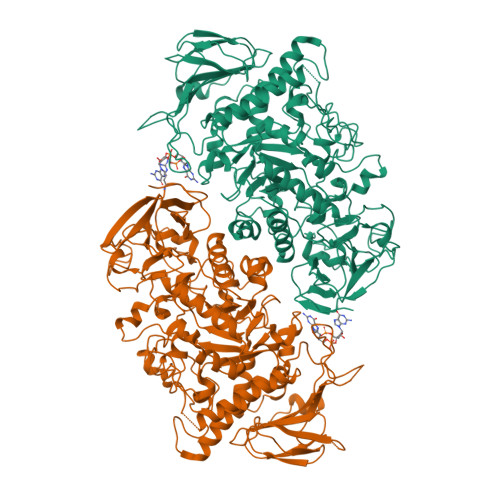
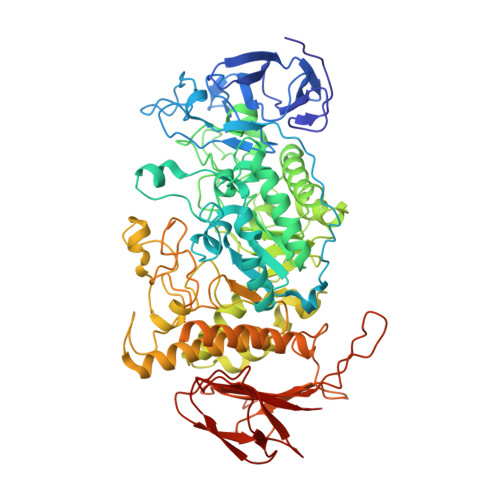
Streptomyces are our principal source of antibiotics, which they generate concomitant with a complex developmental transition from vegetative hyphae to spores. c-di-GMP acts as a linchpin in this transition by binding and regulating the key developmental regulators, BldD and WhiG. Here we show that c-di-GMP also binds the glycogen-debranching-enzyme, GlgX, uncovering a direct link between c-di-GMP and glycogen metabolism in bacteria. Further, we show c-di-GMP binding is required for GlgX activity. We describe structures of apo and c-di-GMP-bound GlgX and, strikingly, their comparison shows c-di-GMP induces long-range conformational changes, reorganizing the catalytic pocket to an active state. Glycogen is an important glucose storage compound that enables animals to cope with starvation and stress. Our in vivo studies reveal the important biological role of GlgX in Streptomyces glucose availability control. Overall, we identify a function of c-di-GMP in controlling energy storage metabolism in bacteria, which is widespread in Actinobacteria.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, 27710, USA. maria.schumacher@duke.edu.