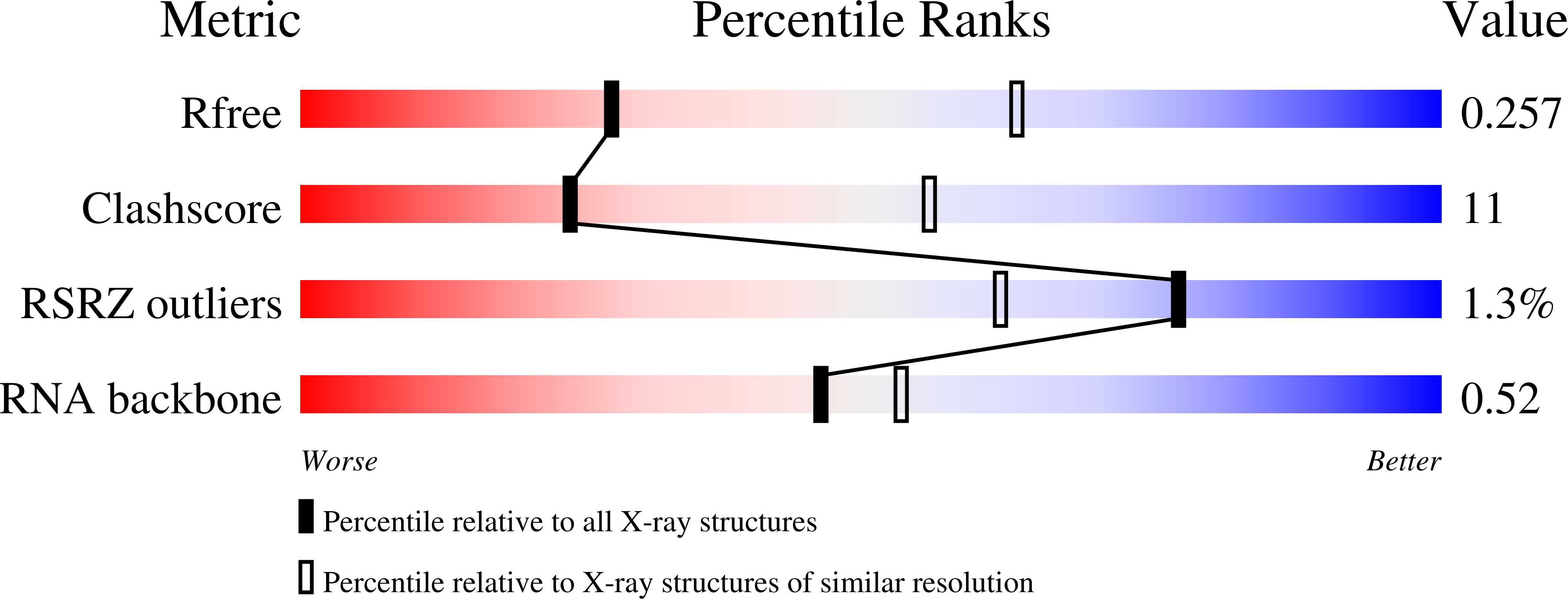SHAPE-enabled fragment-based ligand discovery for RNA.
Zeller, M.J., Favorov, O., Li, K., Nuthanakanti, A., Hussein, D., Michaud, A., Lafontaine, D.A., Busan, S., Serganov, A., Aube, J., Weeks, K.M.(2022) Proc Natl Acad Sci U S A 119: e2122660119-e2122660119
- PubMed: 35561226
- DOI: https://doi.org/10.1073/pnas.2122660119
- Primary Citation of Related Structures:
7TZR, 7TZS, 7TZT, 7TZU - PubMed Abstract:
The transcriptome represents an attractive but underused set of targets for small-molecule ligands. Here, we devise a technology that leverages fragment-based screening and SHAPE-MaP RNA structure probing to discover small-molecule fragments that bind an RNA structure of interest. We identified fragments and cooperatively binding fragment pairs that bind to the thiamine pyrophosphate (TPP) riboswitch with millimolar to micromolar affinities. We then used structure-activity relationship information to efficiently design a linked-fragment ligand, with no resemblance to the native ligand, with high ligand efficiency and druglikeness, that binds to the TPP thiM riboswitch with high nanomolar affinity and that modulates RNA conformation during cotranscriptional folding. Principles from this work are broadly applicable, leveraging cooperativity and multisite binding, for developing high-quality ligands for diverse RNA targets.
Organizational Affiliation:
Department of Chemistry, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599.