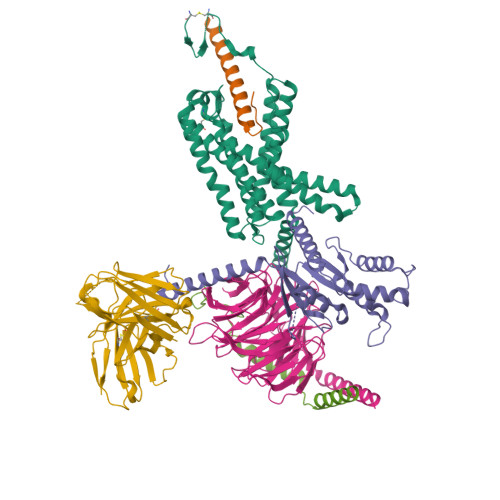
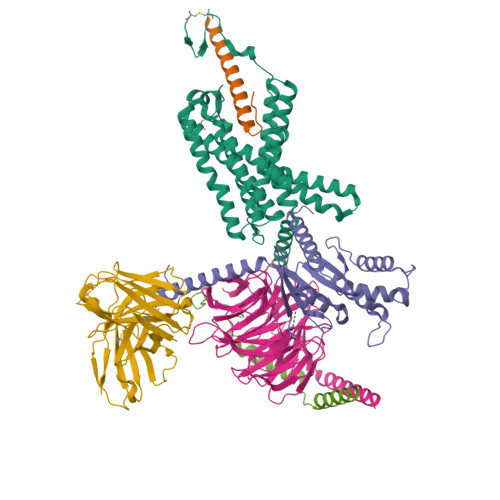
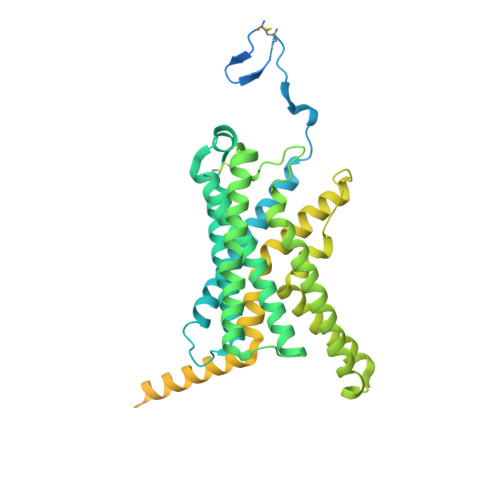
Structure insights into selective coupling of G protein subtypes by a class B G protein-coupled receptor.
Zhao, L.H., Lin, J., Ji, S.Y., Zhou, X.E., Mao, C., Shen, D.D., He, X., Xiao, P., Sun, J., Melcher, K., Zhang, Y., Yu, X., Xu, H.E.(2022) Nat Commun 13: 6670-6670
- PubMed: 36335102
- DOI: https://doi.org/10.1038/s41467-022-33851-3
- Primary Citation of Related Structures:
7TRY, 7TS0 - PubMed Abstract:
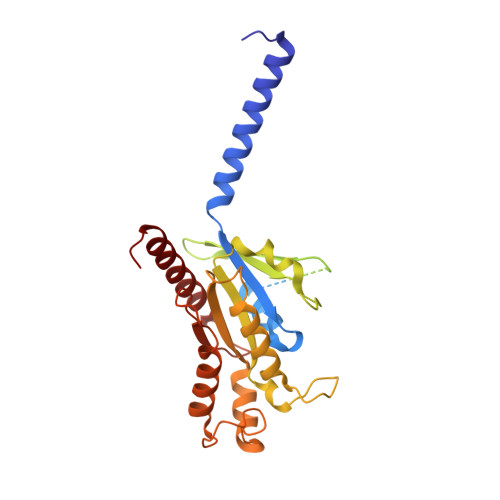
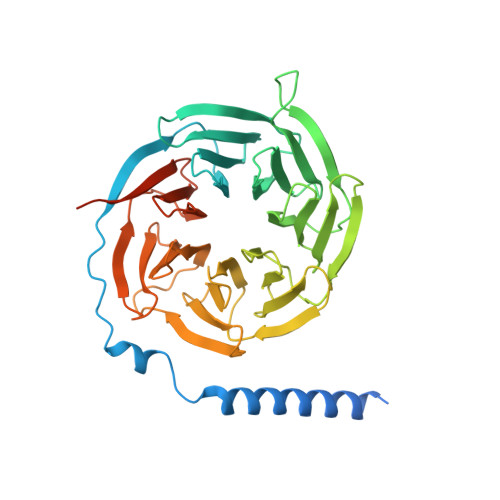
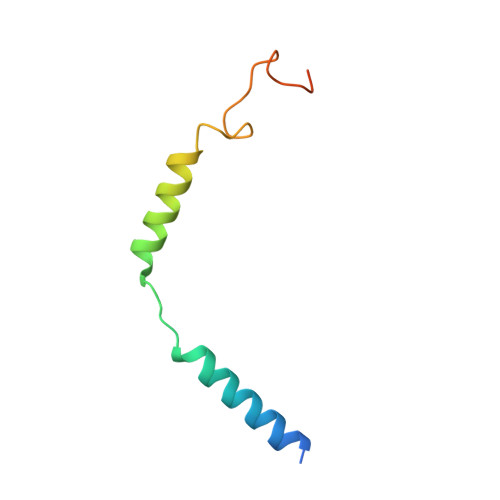
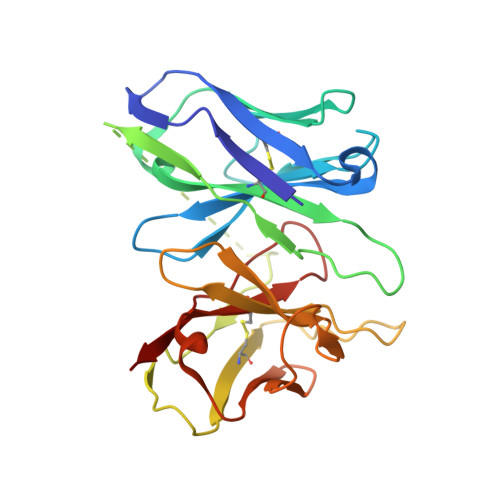
The ability to couple with multiple G protein subtypes, such as G s , G i/o , or G q/11 , by a given G protein-coupled receptor (GPCR) is critical for many physiological processes. Over the past few years, the cryo-EM structures for all 15 members of the medically important class B GPCRs, all in complex with G s protein, have been determined. However, no structure of class B GPCRs with G q/11 has been solved to date, limiting our understanding of the precise mechanisms of G protein coupling selectivity. Here we report the structures of corticotropin releasing factor receptor 2 (CRF2R) bound to Urocortin 1 (UCN1), coupled with different classes of heterotrimeric G proteins, G 11 and G o . We compare these structures with the structure of CRF2R in complex with G s to uncover the structural differences that determine the selective coupling of G protein subtypes by CRF2R. These results provide important insights into the structural basis for the ability of CRF2R to couple with multiple G protein subtypes.
Organizational Affiliation:
The CAS Key Laboratory of Receptor Research, Shanghai Institute of Materia Medica, Chinese Academy of Sciences, Shanghai, 201203, China. zhaolihuawendy@simm.ac.cn.