Facile repurposing of peptide-MHC-restricted antibodies for cancer immunotherapy.
Yang, X., Nishimiya, D., Lochte, S., Jude, K.M., Borowska, M., Savvides, C.S., Dougan, M., Su, L., Zhao, X., Piehler, J., Garcia, K.C.(2023) Nat Biotechnol 41: 932-943
- PubMed: 36593402
- DOI: https://doi.org/10.1038/s41587-022-01567-w
- Primary Citation of Related Structures:
7TR4 - PubMed Abstract:
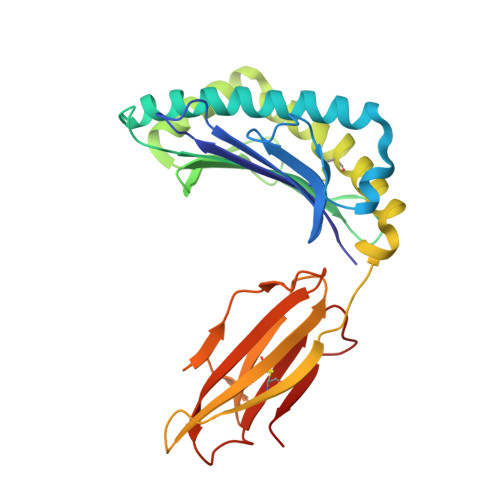
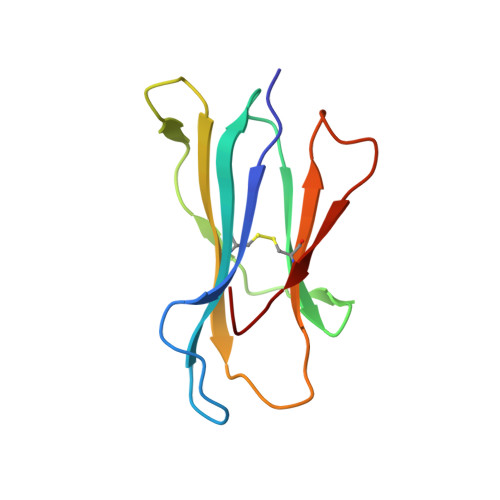
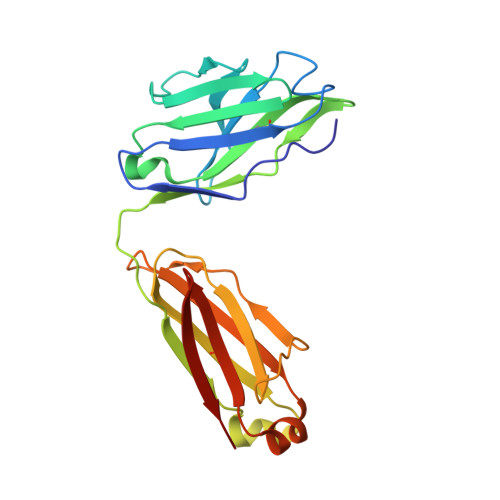
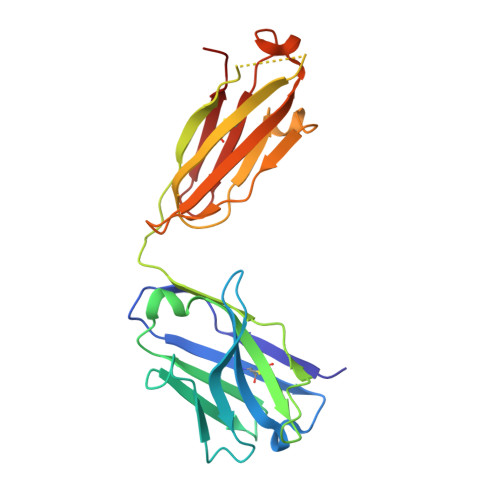
Monoclonal antibodies (Abs) that recognize major histocompatability complex (MHC)-presented tumor antigens in a manner similar to T cell receptors (TCRs) have great potential as cancer immunotherapeutics. However, isolation of 'TCR-mimic' (TCRm) Abs is laborious because Abs have not evolved the structurally nuanced peptide-MHC restriction of αβ-TCRs. Here, we present a strategy for rapid isolation of highly peptide-specific and 'MHC-restricted' Abs by re-engineering preselected Abs that engage peptide-MHC in a manner structurally similar to that of conventional αβ-TCRs. We created structure-based libraries focused on the peptide-interacting residues of TCRm Ab complementarity-determining region (CDR) loops, and rapidly generated MHC-restricted Abs to both mouse and human tumor antigens that specifically killed target cells when formatted as IgG, bispecific T cell engager (BiTE) and chimeric antigen receptor-T (CAR-T). Crystallographic analysis of one selected pMHC-restricted Ab revealed highly peptide-specific recognition, validating the engineering strategy. This approach can yield tumor antigen-specific antibodies in several weeks, potentially enabling rapid clinical translation.
Organizational Affiliation:
Departments of Molecular and Cellular Physiology and Structural Biology, Stanford University School of Medicine, Stanford, CA, USA.