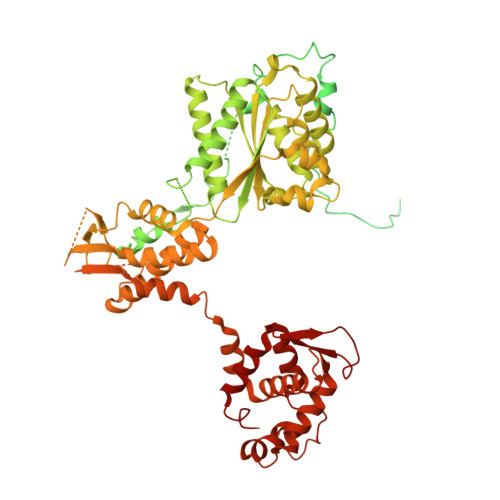
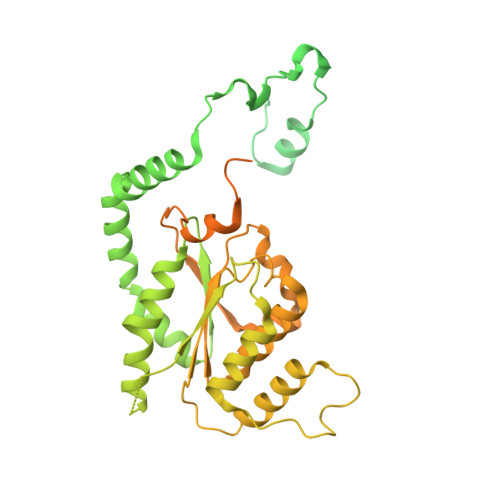
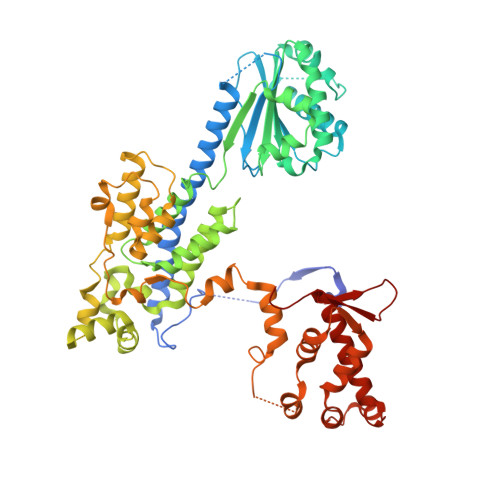
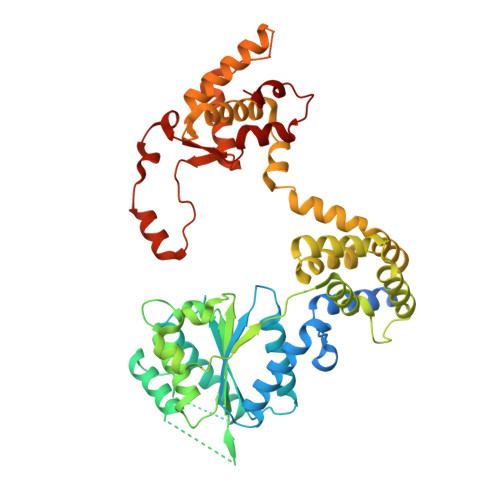
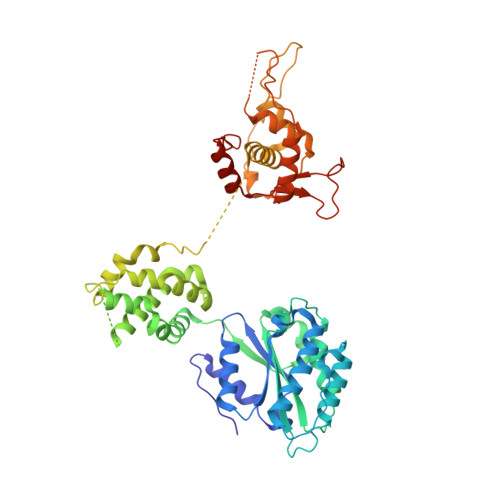
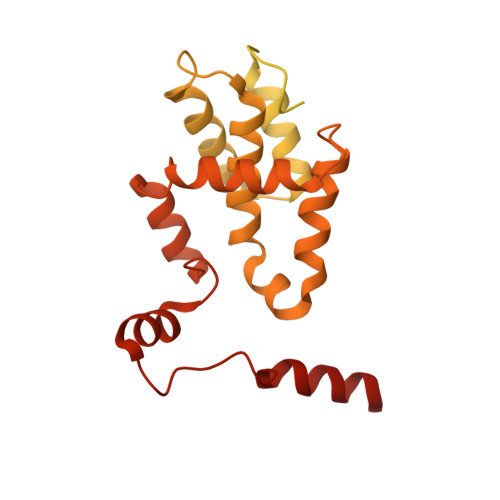
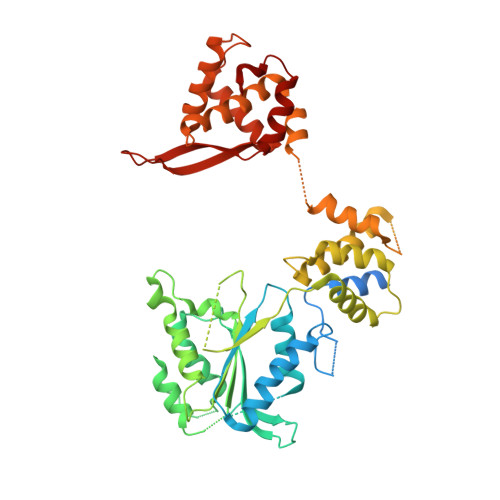
A mechanism of origin licensing control through autoinhibition of S. cerevisiae ORC·DNA·Cdc6.
Schmidt, J.M., Yang, R., Kumar, A., Hunker, O., Seebacher, J., Bleichert, F.(2022) Nat Commun 13: 1059-1059
- PubMed: 35217664
- DOI: https://doi.org/10.1038/s41467-022-28695-w
- Primary Citation of Related Structures:
7TJF, 7TJH, 7TJI, 7TJJ, 7TJK - PubMed Abstract:
The coordinated action of multiple replicative helicase loading factors is needed for the licensing of replication origins prior to DNA replication. Binding of the Origin Recognition Complex (ORC) to DNA initiates the ATP-dependent recruitment of Cdc6, Cdt1 and Mcm2-7 loading, but the structural details for timely ATPase site regulation and for how loading can be impeded by inhibitory signals, such as cyclin-dependent kinase phosphorylation, are unknown. Using cryo-electron microscopy, we have determined several structures of S. cerevisiae ORC·DNA·Cdc6 intermediates at 2.5-2.7 Å resolution. These structures reveal distinct ring conformations of the initiator·co-loader assembly and inactive ATPase site configurations for ORC and Cdc6. The Orc6 N-terminal domain laterally engages the ORC·Cdc6 ring in a manner that is incompatible with productive Mcm2-7 docking, while deletion of this Orc6 region alleviates the CDK-mediated inhibition of Mcm7 recruitment. Our findings support a model in which Orc6 promotes the assembly of an autoinhibited ORC·DNA·Cdc6 intermediate to block origin licensing in response to CDK phosphorylation and to avert DNA re-replication.
Organizational Affiliation:
Friedrich Miescher Institute for Biomedical Research, Basel, 4058, Switzerland.