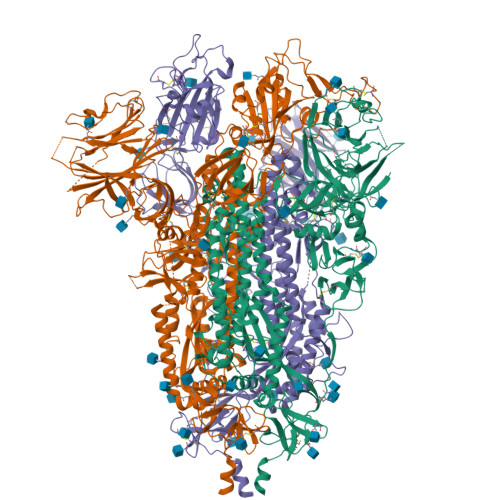
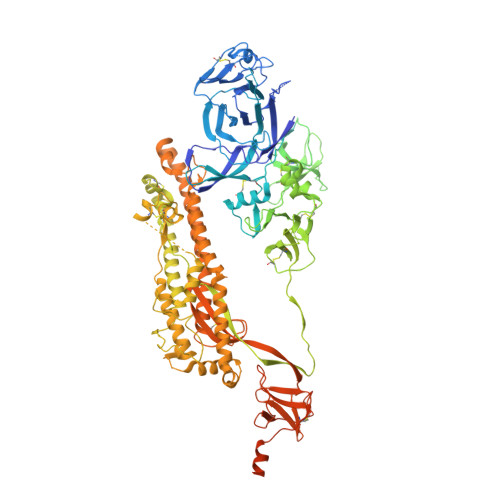
Cryo-EM structure of the SARS-CoV-2 Omicron spike.
Cerutti, G., Guo, Y., Liu, L., Liu, L., Zhang, Z., Luo, Y., Huang, Y., Wang, H.H., Ho, D.D., Sheng, Z., Shapiro, L.(2022) Cell Rep 38: 110428-110428
- PubMed: 35172173
- DOI: https://doi.org/10.1016/j.celrep.2022.110428
- Primary Citation of Related Structures:
7THK - PubMed Abstract:
The recently reported B.1.1.529 Omicron variant of severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) includes 34 mutations in the spike protein relative to the Wuhan strain, including 15 mutations in the receptor-binding domain (RBD). Functional studies have shown Omicron to substantially escape the activity of many SARS-CoV-2-neutralizing antibodies. Here, we report a 3.1 Å-resolution cryoelectron microscopy (cryo-EM) structure of the Omicron spike protein ectodomain. The structure depicts a spike that is exclusively in the 1-RBD-up conformation with high mobility of RBD. Many mutations cause steric clashes and/or altered interactions at antibody-binding surfaces, whereas others mediate changes of the spike structure in local regions to interfere with antibody recognition. Overall, the structure of the Omicron spike reveals how mutations alter its conformation and explains its extraordinary ability to evade neutralizing antibodies.
Organizational Affiliation:
Department of Biochemistry and Molecular Biophysics, Columbia University, New York, NY 10032, USA; Zuckerman Mind Brain Behavior Institute, Columbia University, New York, NY 10027, USA.