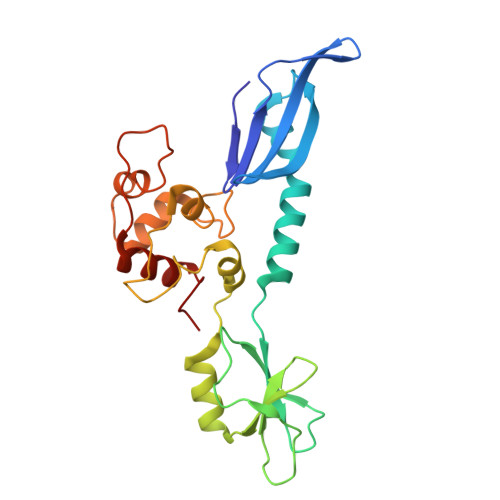
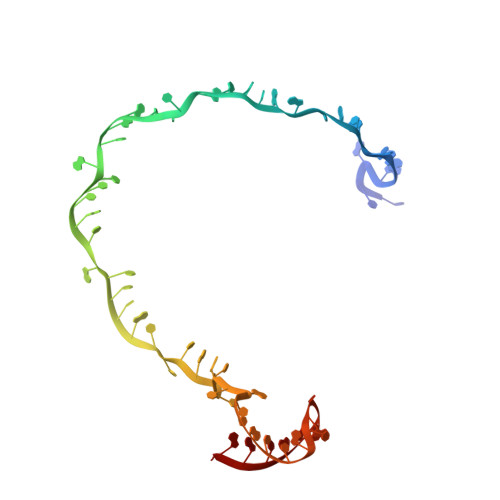
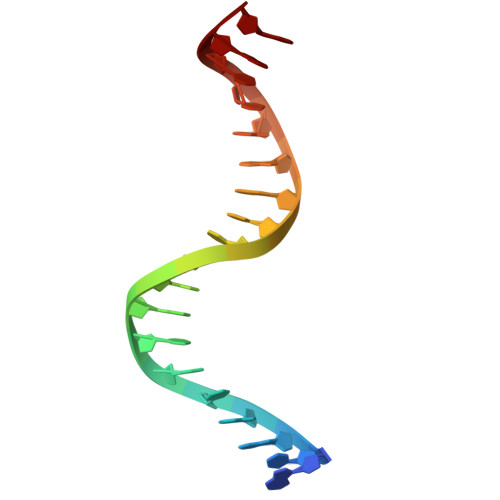
Structural basis of AcrIF24 as an anti-CRISPR protein and transcriptional suppressor.
Mukherjee, I.A., Gabel, C., Noinaj, N., Bondy-Denomy, J., Chang, L.(2022) Nat Chem Biol 18: 1417-1424
- PubMed: 36163386
- DOI: https://doi.org/10.1038/s41589-022-01137-w
- Primary Citation of Related Structures:
7T3J, 7T3K, 7T3L, 7TAW, 7TAX - PubMed Abstract:
Anti-CRISPR (Acr) proteins are encoded by phages to inactivate CRISPR-Cas systems of bacteria and archaea and are used to enhance the CRISPR toolbox for genome editing. Here we report the structure and mechanism of AcrIF24, an Acr protein that inhibits the type I-F CRISPR-Cas system from Pseudomonas aeruginosa. AcrIF24 is a homodimer that associates with two copies of the surveillance complex (Csy) and prevents the hybridization between CRISPR RNA and target DNA. Furthermore, AcrIF24 functions as an anti-CRISPR-associated (Aca) protein to repress the transcription of the acrIF23-acrIF24 operon. Alone or in complex with Csy, AcrIF24 is capable of binding to the acrIF23-acrIF24 promoter DNA with nanomolar affinity. The structure of a Csy-AcrIF24-promoter DNA complex at 2.7 Å reveals the mechanism for transcriptional suppression. Our results reveal that AcrIF24 functions as an Acr-Aca fusion protein, and they extend understanding of the diverse mechanisms used by Acr proteins.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN, USA.