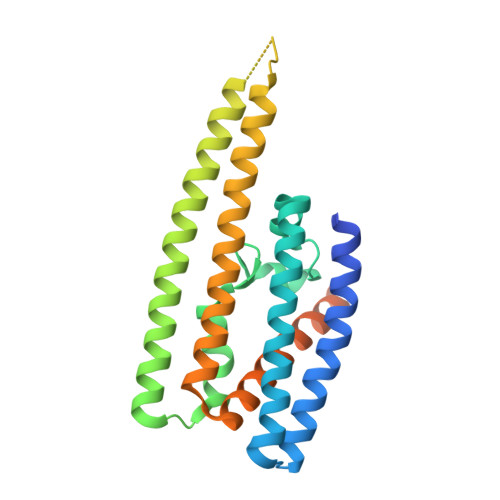
Structural basis of NPR1 in activating plant immunity.
Kumar, S., Zavaliev, R., Wu, Q., Zhou, Y., Cheng, J., Dillard, L., Powers, J., Withers, J., Zhao, J., Guan, Z., Borgnia, M.J., Bartesaghi, A., Dong, X., Zhou, P.(2022) Nature 605: 561-566
- PubMed: 35545668
- DOI: https://doi.org/10.1038/s41586-022-04699-w
- Primary Citation of Related Structures:
7MK2, 7MK3, 7TAC, 7TAD, 7TAE - PubMed Abstract:
NPR1 is a master regulator of the defence transcriptome induced by the plant immune signal salicylic acid 1-4 . Despite the important role of NPR1 in plant immunity 5-7 , understanding of its regulatory mechanisms has been hindered by a lack of structural information. Here we report cryo-electron microscopy and crystal structures of Arabidopsis NPR1 and its complex with the transcription factor TGA3. Cryo-electron microscopy analysis reveals that NPR1 is a bird-shaped homodimer comprising a central Broad-complex, Tramtrack and Bric-à-brac (BTB) domain, a BTB and carboxyterminal Kelch helix bundle, four ankyrin repeats and a disordered salicylic-acid-binding domain. Crystal structure analysis reveals a unique zinc-finger motif in BTB for interacting with ankyrin repeats and mediating NPR1 oligomerization. We found that, after stimulation, salicylic-acid-induced folding and docking of the salicylic-acid-binding domain onto ankyrin repeats is required for the transcriptional cofactor activity of NPR1, providing a structural explanation for a direct role of salicylic acid in regulating NPR1-dependent gene expression. Moreover, our structure of the TGA3 2 -NPR1 2 -TGA3 2 complex, DNA-binding assay and genetic data show that dimeric NPR1 activates transcription by bridging two fatty-acid-bound TGA3 dimers to form an enhanceosome. The stepwise assembly of the NPR1-TGA complex suggests possible hetero-oligomeric complex formation with other transcription factors, revealing how NPR1 reprograms the defence transcriptome.
Organizational Affiliation:
Department of Biochemistry, Duke University School of Medicine, Durham, NC, USA.