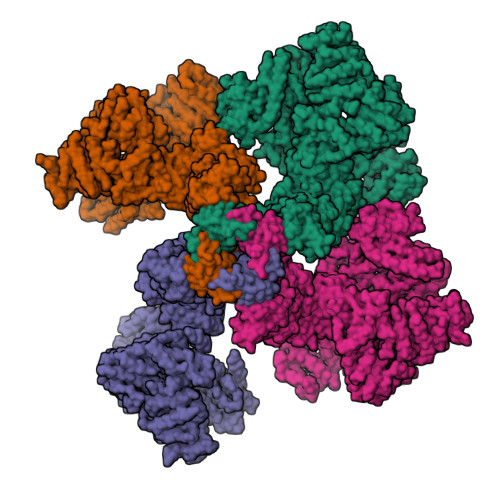
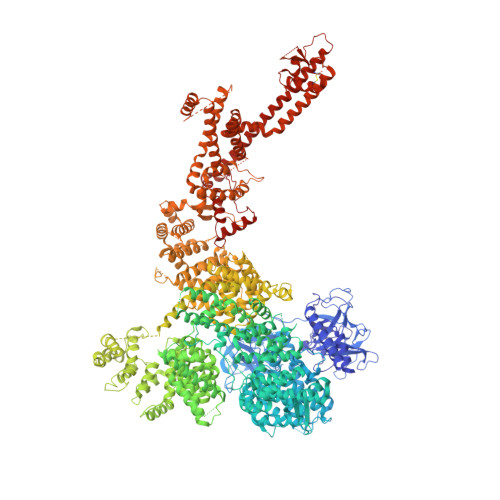
Structural basis for activation and gating of IP 3 receptors.
Schmitz, E.A., Takahashi, H., Karakas, E.(2022) Nat Commun 13: 1408-1408
- PubMed: 35301323
- DOI: https://doi.org/10.1038/s41467-022-29073-2
- Primary Citation of Related Structures:
7T3P, 7T3Q, 7T3R, 7T3T, 7T3U - PubMed Abstract:
A pivotal component of the calcium (Ca 2+ ) signaling toolbox in cells is the inositol 1,4,5-triphosphate (IP 3 ) receptor (IP 3 R), which mediates Ca 2+ release from the endoplasmic reticulum (ER), controlling cytoplasmic and organellar Ca 2+ concentrations. IP 3 Rs are co-activated by IP 3 and Ca 2+ , inhibited by Ca 2+ at high concentrations, and potentiated by ATP. However, the underlying molecular mechanisms are unclear. Here we report cryo-electron microscopy (cryo-EM) structures of human type-3 IP 3 R obtained from a single dataset in multiple gating conformations: IP 3 -ATP bound pre-active states with closed channels, IP 3 -ATP-Ca 2+ bound active state with an open channel, and IP 3 -ATP-Ca 2+ bound inactive state with a closed channel. The structures demonstrate how IP 3 -induced conformational changes prime the receptor for activation by Ca 2+ , how Ca 2+ binding leads to channel opening, and how ATP modulates the activity, providing insights into the long-sought questions regarding the molecular mechanism underpinning receptor activation and gating.
Organizational Affiliation:
Department of Molecular Physiology and Biophysics, Vanderbilt University, School of Medicine, Nashville, TN, 37232, USA.