An allosteric inhibitor of sirtuin 2 deacetylase activity exhibits broad-spectrum antiviral activity.
Roche, K.L., Remiszewski, S., Todd, M.J., Kulp 3rd, J.L., Tang, L., Welsh, A.V., Barry, A.P., De, C., Reiley, W.W., Wahl, A., Garcia, J.V., Luftig, M.A., Shenk, T., Tonra, J.R., Murphy, E.A., Chiang, L.W.(2023) J Clin Invest 133
- PubMed: 37317966
- DOI: https://doi.org/10.1172/JCI158978
- Primary Citation of Related Structures:
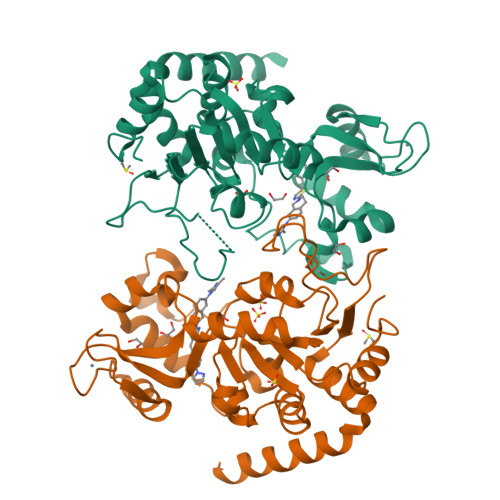
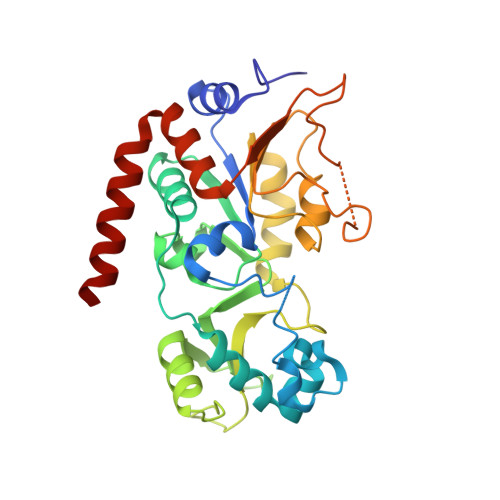
7T1D - PubMed Abstract:
Most drugs used to treat viral disease target a virus-coded product. They inhibit a single virus or virus family, and the pathogen can readily evolve resistance. Host-targeted antivirals can overcome these limitations. The broad-spectrum activity achieved by host targeting can be especially useful in combating emerging viruses and for treatment of diseases caused by multiple viral pathogens, such as opportunistic agents in immunosuppressed patients. We have developed a family of compounds that modulate sirtuin 2, an NAD+-dependent deacylase, and now report the properties of a member of that family, FLS-359. Biochemical and x-ray structural studies show that the drug binds to sirtuin 2 and allosterically inhibits its deacetylase activity. FLS-359 inhibits the growth of RNA and DNA viruses, including members of the coronavirus, orthomyxovirus, flavivirus, hepadnavirus, and herpesvirus families. FLS-359 acts at multiple levels to antagonize cytomegalovirus replication in fibroblasts, causing modest reductions in viral RNAs and DNA, together with a much greater reduction in infectious progeny, and it exhibits antiviral activity in humanized mouse models of infection. Our results highlight the potential of sirtuin 2 inhibitors as broad-spectrum antivirals and set the stage for further understanding of how host epigenetic mechanisms impact the growth and spread of viral pathogens.
Organizational Affiliation:
Evrys Bio LLC, Pennsylvania Biotechnology Center, Doylestown, Pennsylvania, USA.