Temporal and spatial resolution of distal protein motions that activate hydrogen tunneling in soybean lipoxygenase.
Zaragoza, J.P.T., Offenbacher, A.R., Hu, S., Gee, C.L., Firestein, Z.M., Minnetian, N., Deng, Z., Fan, F., Iavarone, A.T., Klinman, J.P.(2023) Proc Natl Acad Sci U S A 120: e2211630120-e2211630120
- PubMed: 36867685
- DOI: https://doi.org/10.1073/pnas.2211630120
- Primary Citation of Related Structures:
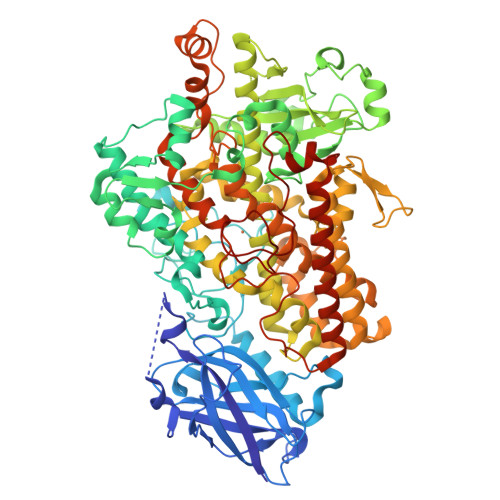
7SOI, 7SOJ - PubMed Abstract:
The enzyme soybean lipoxygenase (SLO) provides a prototype for deep tunneling mechanisms in hydrogen transfer catalysis. This work combines room temperature X-ray studies with extended hydrogen-deuterium exchange experiments to define a catalytically-linked, radiating cone of aliphatic side chains that connects an active site iron center of SLO to the protein-solvent interface. Employing eight variants of SLO that have been appended with a fluorescent probe at the identified surface loop, nanosecond fluorescence Stokes shifts have been measured. We report a remarkable identity of the energies of activation ( E a ) for the Stokes shifts decay rates and the millisecond C-H bond cleavage step that is restricted to side chain mutants within an identified thermal network. These findings implicate a direct coupling of distal protein motions surrounding the exposed fluorescent probe to active site motions controlling catalysis. While the role of dynamics in enzyme function has been predominantly attributed to a distributed protein conformational landscape, the presented data implicate a thermally initiated, cooperative protein reorganization that occurs on a timescale faster than nanosecond and represents the enthalpic barrier to the reaction of SLO.
Organizational Affiliation:
California Institute for Quantitative Biosciences, University of California Berkeley, Berkeley, CA 94720.