Structural insights into binding of therapeutic channel blockers in NMDA receptors.
Chou, T.H., Epstein, M., Michalski, K., Fine, E., Biggin, P.C., Furukawa, H.(2022) Nat Struct Mol Biol 29: 507-518
- PubMed: 35637422
- DOI: https://doi.org/10.1038/s41594-022-00772-0
- Primary Citation of Related Structures:
7SAA, 7SAB, 7SAC, 7SAD - PubMed Abstract:
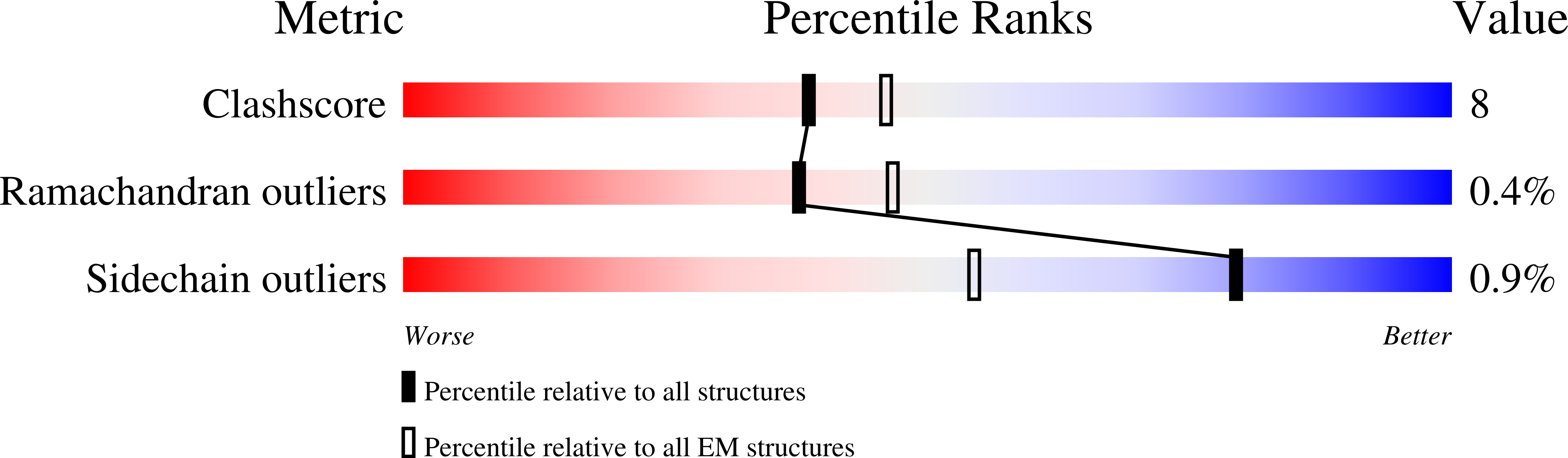
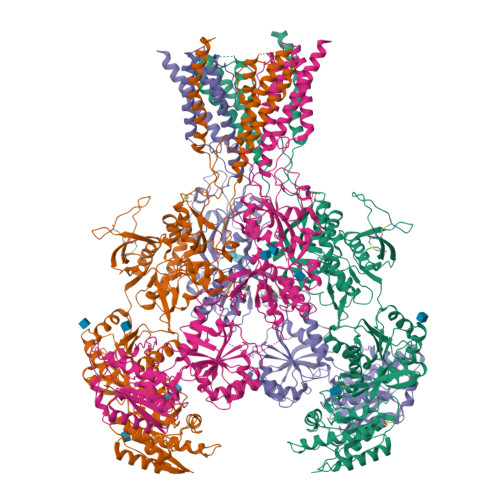
Excitatory signaling mediated by N-methyl-D-aspartate receptor (NMDAR) is critical for brain development and function, as well as for neurological diseases and disorders. Channel blockers of NMDARs are of medical interest owing to their potential for treating depression, Alzheimer's disease, and epilepsy. However, precise mechanisms underlying binding and channel blockade have remained limited owing to challenges in obtaining high-resolution structures at the binding site within the transmembrane domains. Here, we monitor the binding of three clinically important channel blockers: phencyclidine, ketamine, and memantine in GluN1-2B NMDARs at local resolutions of 2.5-3.5 Å around the binding site using single-particle electron cryo-microscopy, molecular dynamics simulations, and electrophysiology. The channel blockers form different extents of interactions with the pore-lining residues, which control mostly off-speeds but not on-speeds. Our comparative analyses of the three unique NMDAR channel blockers provide a blueprint for developing therapeutic compounds with minimal side effects.
Organizational Affiliation:
W.M. Keck Structural Biology Laboratory, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York, NY, USA.