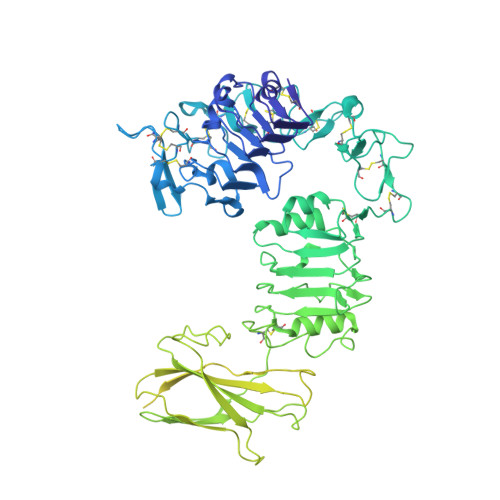
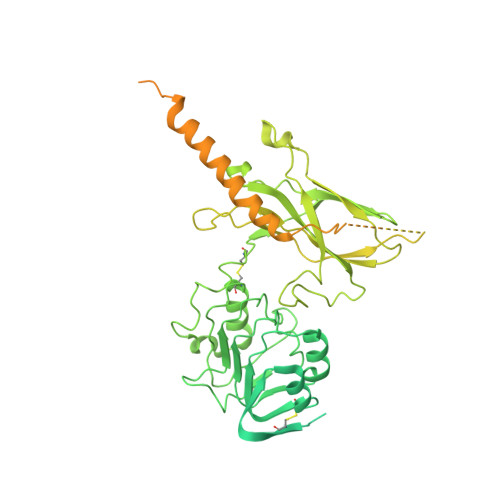
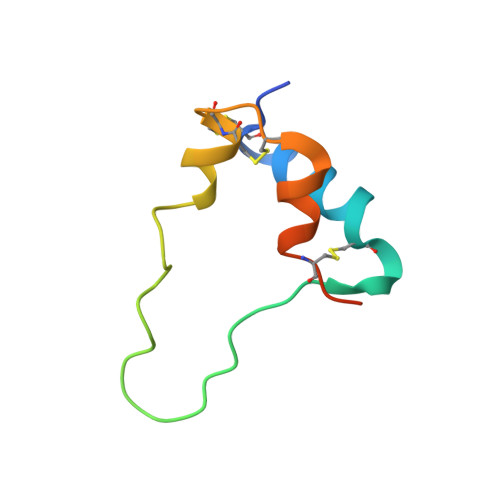
How insulin-like growth factor I binds to a hybrid insulin receptor type 1 insulin-like growth factor receptor.
Xu, Y., Margetts, M.B., Venugopal, H., Menting, J.G., Kirk, N.S., Croll, T.I., Delaine, C., Forbes, B.E., Lawrence, M.C.(2022) Structure 30: 1098-1108.e6
- PubMed: 35660159
- DOI: https://doi.org/10.1016/j.str.2022.05.007
- Primary Citation of Related Structures:
7S0Q, 7S8V - PubMed Abstract:
Monomers of the insulin receptor and type 1 insulin-like growth factor receptor (IGF-1R) can combine stochastically to form heterodimeric hybrid receptors. These hybrid receptors display ligand binding and signaling properties that differ from those of the homodimeric receptors. Here, we describe the cryoelectron microscopy structure of such a hybrid receptor in complex with insulin-like growth factor I (IGF-I). The structure (ca. 3.7 Å resolution) displays a single IGF-I ligand, bound in a similar fashion to that seen for IGFs in complex with IGF-1R. The IGF-I ligand engages the first leucine-rich-repeat domain and cysteine-rich region of the IGF-1R monomer (rather than those of the insulin receptor monomer), consistent with the determinants for IGF binding residing in the IGF-1R cysteine-rich region. The structure broadens our understanding of this receptor family and assists in delineating the key structural motifs involved in binding their respective ligands.
Organizational Affiliation:
WEHI, 1G Royal Parade, Parkville, VIC 3052, Australia; Department of Medical Biology, Faculty of Medicine, Dentistry and Health Sciences, University of Melbourne, Parkville, VIC 3050, Australia.