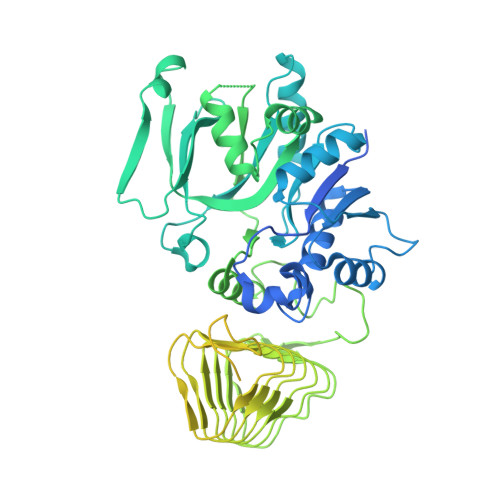
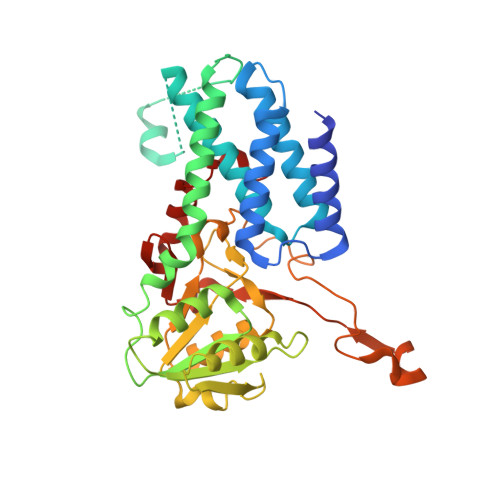
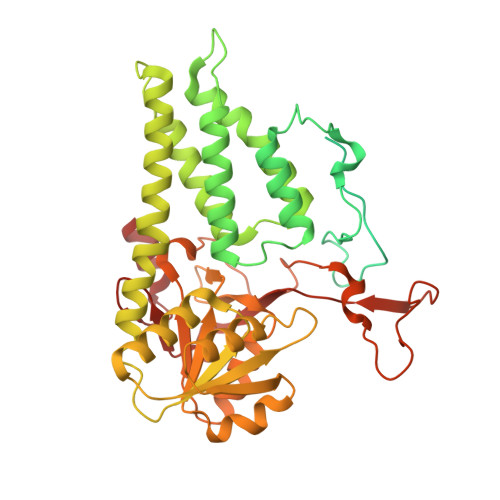
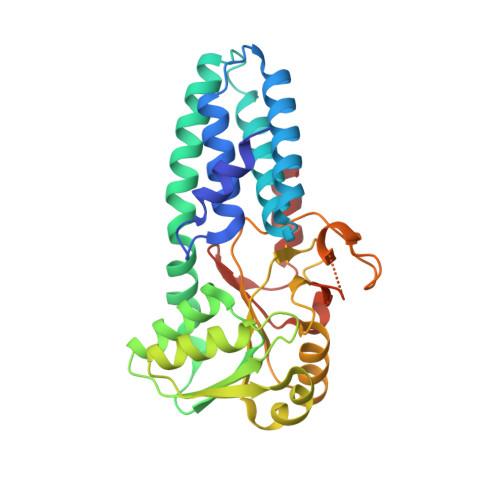
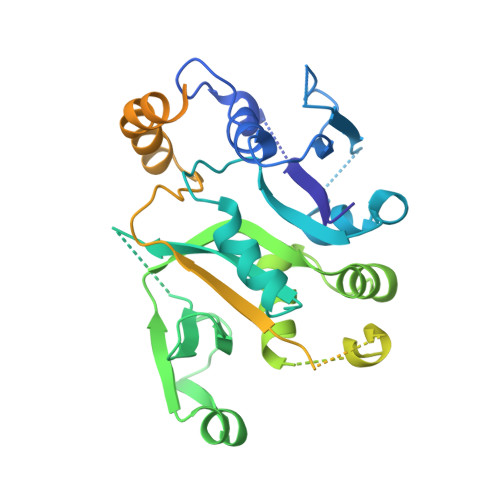
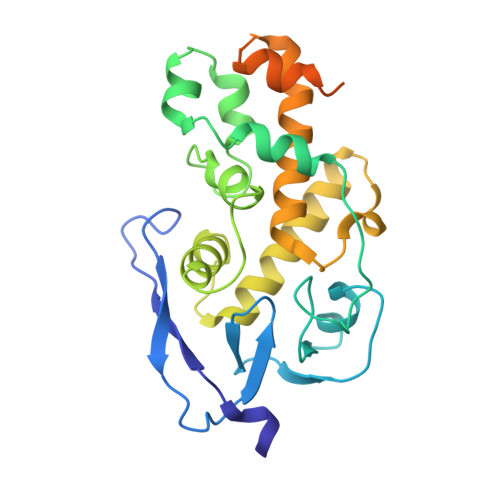
Viral evasion of the integrated stress response through antagonism of eIF2-P binding to eIF2B.
Schoof, M., Wang, L., Cogan, J.Z., Lawrence, R.E., Boone, M., Wuerth, J.D., Frost, A., Walter, P.(2021) Nat Commun 12: 7103-7103
- PubMed: 34876554
- DOI: https://doi.org/10.1038/s41467-021-26164-4
- Primary Citation of Related Structures:
7RLO - PubMed Abstract:
Viral infection triggers activation of the integrated stress response (ISR). In response to viral double-stranded RNA (dsRNA), RNA-activated protein kinase (PKR) phosphorylates the translation initiation factor eIF2, converting it from a translation initiator into a potent translation inhibitor and this restricts the synthesis of viral proteins. Phosphorylated eIF2 (eIF2-P) inhibits translation by binding to eIF2's dedicated, heterodecameric nucleotide exchange factor eIF2B and conformationally inactivating it. We show that the NSs protein of Sandfly Fever Sicilian virus (SFSV) allows the virus to evade the ISR. Mechanistically, NSs tightly binds to eIF2B (K D = 30 nM), blocks eIF2-P binding, and rescues eIF2B GEF activity. Cryo-EM structures demonstrate that SFSV NSs and eIF2-P directly compete, with the primary NSs contacts to eIF2Bα mediated by five 'aromatic fingers'. NSs binding preserves eIF2B activity by maintaining eIF2B's conformation in its active A-State.
Organizational Affiliation:
Howard Hughes Medical Institute, University of California at San Francisco, San Francisco, CA, USA.