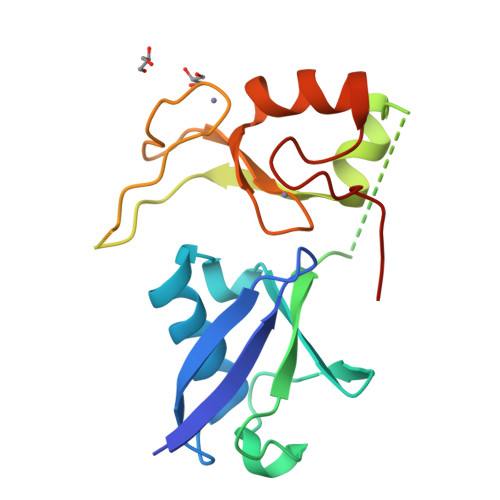
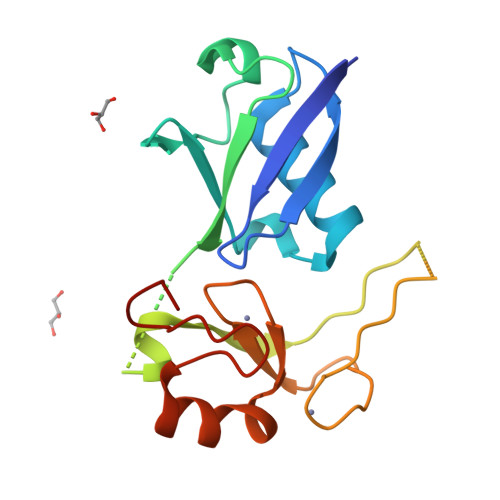
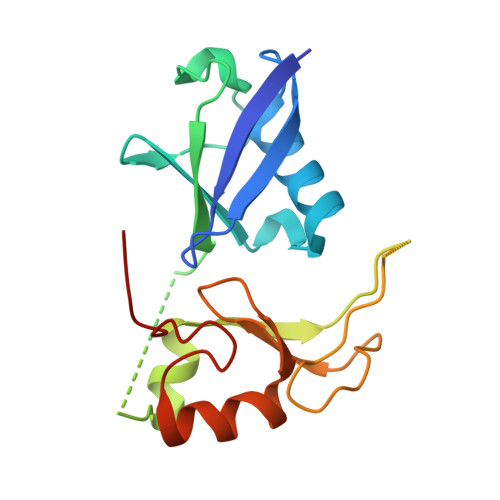
Ubiquitin and a charged loop regulate the ubiquitin E3 ligase activity of Ark2C.
Paluda, A., Middleton, A.J., Rossig, C., Mace, P.D., Day, C.L.(2022) Nat Commun 13: 1181-1181
- PubMed: 35246518
- DOI: https://doi.org/10.1038/s41467-022-28782-y
- Primary Citation of Related Structures:
7R70, 7R71 - PubMed Abstract:
A large family of E3 ligases that contain both substrate recruitment and RING domains confer specificity within the ubiquitylation cascade. Regulation of RING E3s depends on modulating their ability to stabilise the RING bound E2~ubiquitin conjugate in the activated (or closed) conformation. Here we report the structure of the Ark2C RING bound to both a regulatory ubiquitin molecule and an activated E2~ubiquitin conjugate. The structure shows that the RING domain and non-covalently bound ubiquitin molecule together make contacts that stabilise the activated conformation of the conjugate, revealing why ubiquitin is a key regulator of Ark2C activity. We also identify a charged loop N-terminal to the RING domain that enhances activity by interacting with both the regulatory ubiquitin and ubiquitin conjugated to the E2. In addition, the structure suggests how Lys48-linked ubiquitin chains might be assembled by Ark2C and UbcH5b. Together this study identifies features common to RING E3s, as well elements that are unique to Ark2C and related E3s, which enhance assembly of ubiquitin chains.
Organizational Affiliation:
Biochemistry Department, School of Biomedical Sciences, University of Otago, Dunedin, 9054, New Zealand.