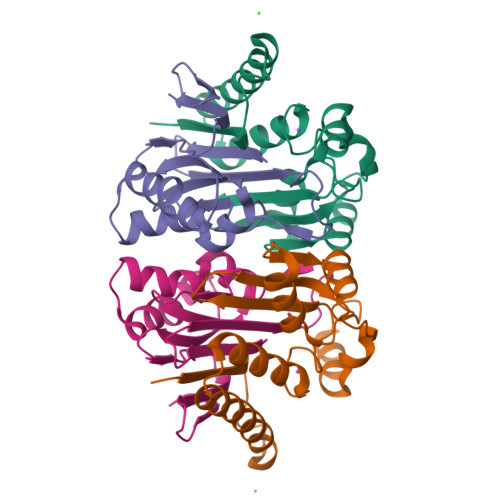
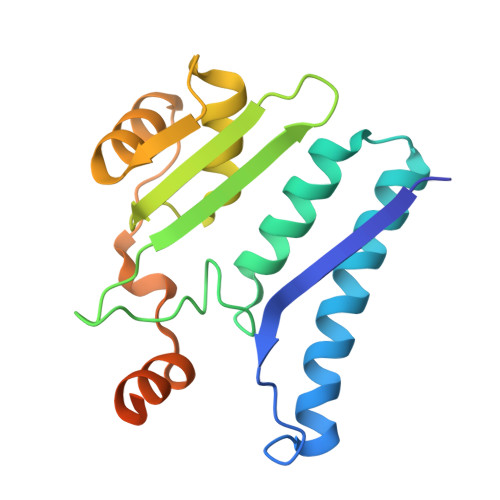
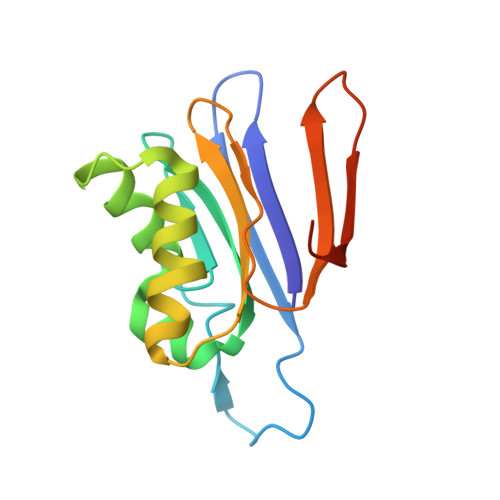
Structural and biophysical studies of new L-asparaginase variants: lessons from random mutagenesis of the prototypic Escherichia coli Ntn-amidohydrolase.
Loch, J.I., Klonecka, A., Kadziolka, K., Bonarek, P., Barciszewski, J., Imiolczyk, B., Brzezinski, K., Gilski, M., Jaskolski, M.(2022) Acta Crystallogr D Struct Biol 78: 911-926
- PubMed: 35775990
- DOI: https://doi.org/10.1107/S2059798322005691
- Primary Citation of Related Structures:
7QQ8, 7QSF, 7QTC, 7QVR, 7QY6, 7QYM, 7QYX, 7R1G, 7R5C - PubMed Abstract:
This work reports the results of random mutagenesis of the Escherichia coli class 2 L-asparaginase EcAIII belonging to the Ntn-hydrolase family. New variants of EcAIII were studied using structural, biophysical and bioinformatic methods. Activity tests revealed that the L-asparaginase activity is abolished in all analyzed mutants with the absence of Arg207, but some of them retained the ability to undergo the autoproteolytic maturation process. The results of spectroscopic studies and the determined crystal structures showed that the EcAIII fold is flexible enough to accept different types of mutations; however, these mutations may have a diverse impact on the thermal stability of the protein. The conclusions from the experiments are grouped into six lessons focused on (i) the adaptation of the EcAIII fold to new substitutions, (ii) the role of Arg207 in EcAIII activity, (iii) a network of residues necessary for autoprocessing, (iv) the complexity of the autoprocessing reaction, (v) the conformational changes observed in enzymatically inactive variants and (vi) the cooperativity of the EcAIII dimer subunits. Additionally, the structural requirements (pre-maturation checkpoints) that are necessary for the initiation of the autocleavage of Ntn-hydrolases have been classified. The findings reported in this work provide useful hints that should be considered before planning enzyme-engineering experiments aimed at the design of proteins for therapeutic applications. This is especially important for L-asparaginases that can be utilized in leukemia therapy, as alternative therapeutics are urgently needed to circumvent the severe side effects associated with the currently used enzymes.
Organizational Affiliation:
Department of Crystal Chemistry and Crystal Physics, Faculty of Chemistry, Jagiellonian University, Krakow, Poland.