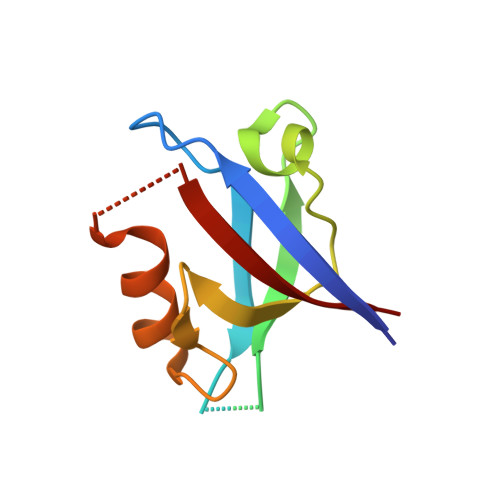
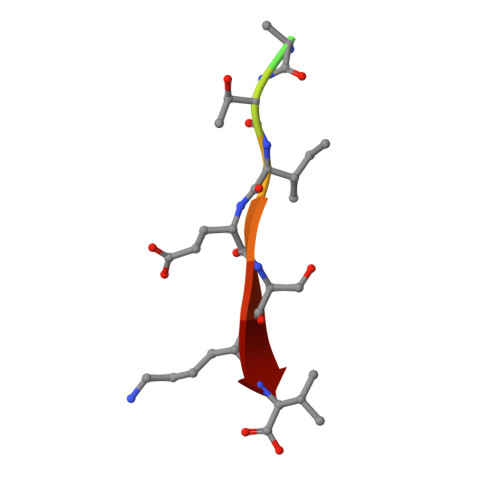
Structural Basis of the Avian Influenza NS1 Protein Interactions with the Cell Polarity Regulator Scribble.
Javorsky, A., Humbert, P.O., Kvansakul, M.(2022) Viruses 14
- PubMed: 35336989
- DOI: https://doi.org/10.3390/v14030583
- Primary Citation of Related Structures:
7QTO, 7QTP, 7QTU - PubMed Abstract:
Scribble is a highly conserved regulator of cell polarity, a process that enables the generation of asymmetry at the cellular and tissue level in higher organisms. Scribble acts in concert with Disc-large (Dlg) and Lethal-2-giant larvae (Lgl) to form the Scribble polarity complex, and its functional dysregulation is associated with poor prognosis during viral infections. Viruses have been shown to interfere with Scribble by targeting Scribble PDZ domains to subvert the network of interactions that enable normal control of cell polarity via Scribble, as well as the localisation of the Scribble module within the cell. The influenza A virus NS1 protein was shown to bind to human Scribble (SCRIB) via its C-terminal PDZ binding motif (PBM). It was reported that the PBM sequence ESEV is a virulence determinant for influenza A virus H5N1 whilst other sequences, such as ESKV, KSEV and RSKV, demonstrated no affinity towards Scribble. We now show, using isothermal titration calorimetry (ITC), that ESKV and KSEV bind to SCRIB PDZ domains and that ESEV unexpectedly displayed an affinity towards all four PDZs and not just a selected few. We then define the structural basis for the interactions of SCRIB PDZ1 domain with ESEV and ESKV PBM motifs, as well as SCRIB PDZ3 with the ESKV PBM motif. These findings will serve as a platform for understanding the role of Scribble PDZ domains and their interactions with different NS1 PBMs and the mechanisms that mediate cell polarity within the context of the pathogenesis of influenza A virus.
Organizational Affiliation:
Department of Biochemistry and Chemistry, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, VIC 3086, Australia.