Structural insight into the Scribble PDZ domains interaction with the oncogenic Human T-cell lymphotrophic virus-1 (HTLV-1) Tax1 PBM.
Javorsky, A., Maddumage, J.C., Mackie, E.R.R., Soares da Costa, T.P., Humbert, P.O., Kvansakul, M.(2023) FEBS J 290: 974-987
- PubMed: 36029163
- DOI: https://doi.org/10.1111/febs.16607
- Primary Citation of Related Structures:
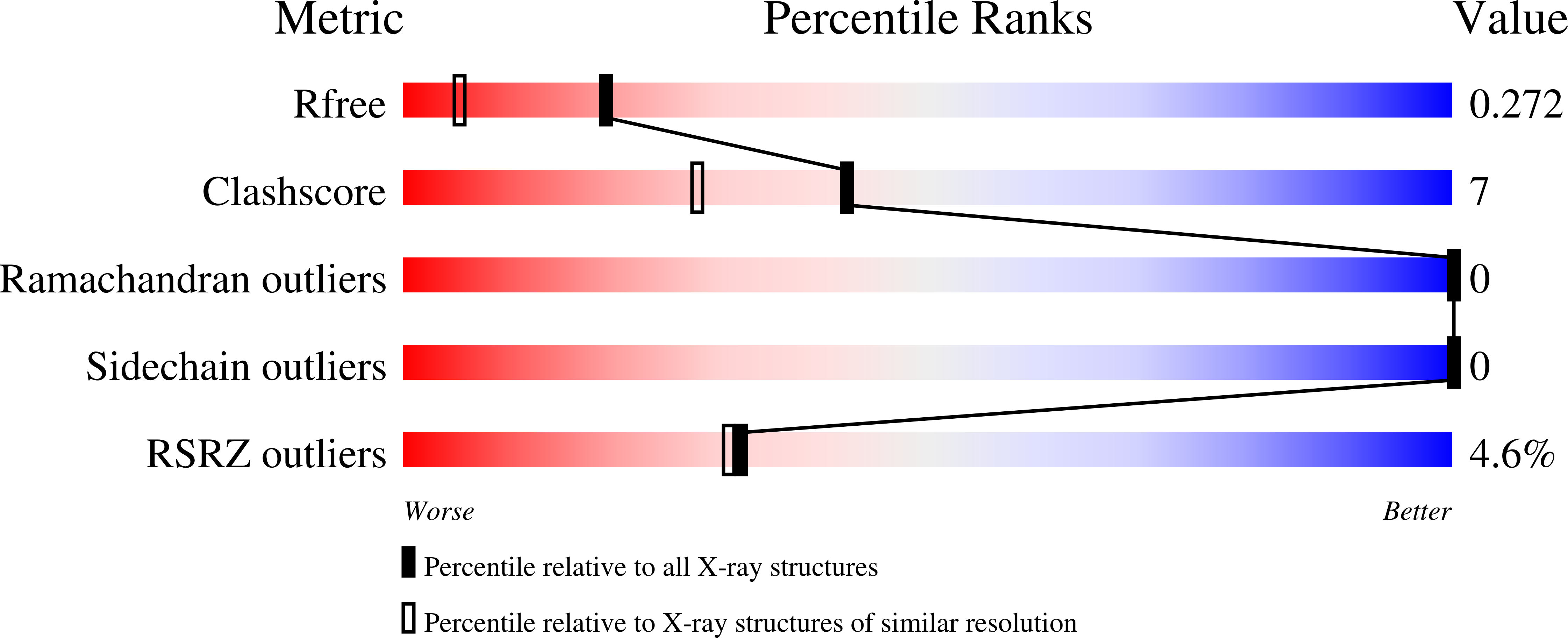
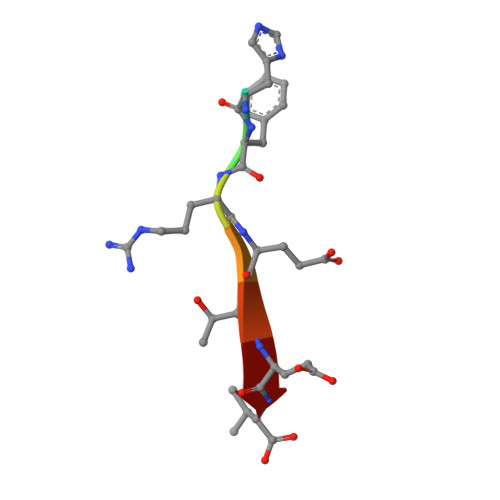
7QRS, 7QRT, 7QS8 - PubMed Abstract:
Scribble (Scrib) is a highly conserved cell polarity regulator that harbours potent tumour suppressor activity and plays an important role in cell migration. Dysregulation of polarity is associated with poor prognosis during viral infections. Human T-cell lymphotrophic virus-1 (HTLV-1) encodes for the oncogenic Tax1 protein, a modulator of the transcription of viral and human proteins that can cause cell cycle dysregulation as well as a loss of genomic integrity. Previous studies established that Scribble interacts with Tax1 via its C-terminal PDZ-binding motif (PBM), leading to aggregation of polarity regulators and subsequent perturbation of host cell adhesion, proliferation, and signalling. Using isothermal titration calorimetry, we now show that all four PDZ domains of Scribble bind to Tax1 PBM. We then determined crystal structures of Scribble PDZ1, PDZ2 and PDZ3 domains bound to Tax1 PBM. Our findings establish a structural basis for Tax1-mediated subversion of Scribble-mediated cell polarity signalling and provide the platform for mechanistic studies to examine Tax1 induced mislocalization of Scribble and the associated changes in cellular architecture and subsequent tumorigenesis.
Organizational Affiliation:
Department of Biochemistry & Chemistry, La Trobe Institute for Molecular Science, La Trobe University, Melbourne, Vic., Australia.