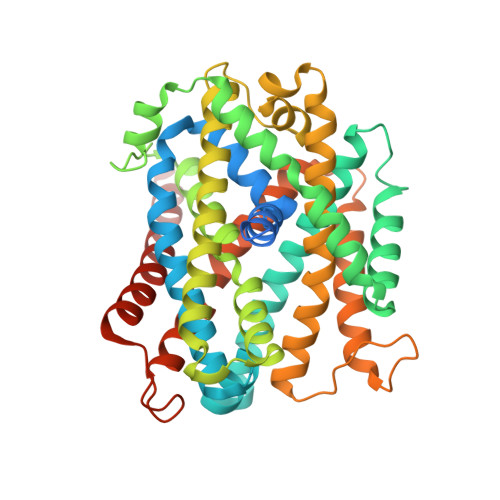
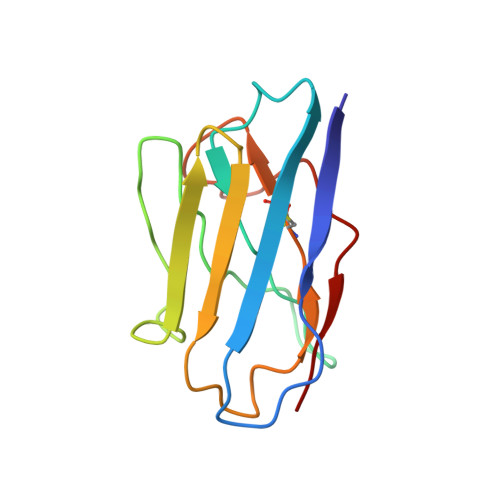
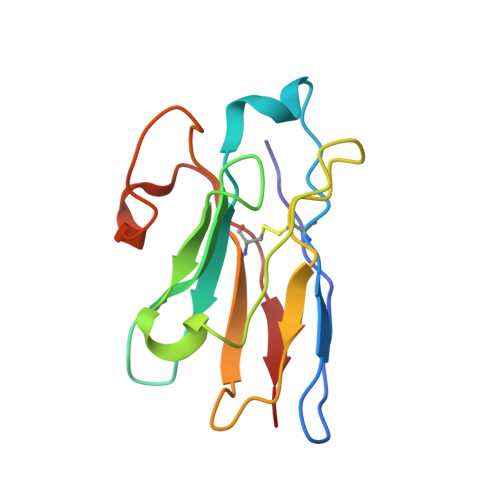
Structural and functional properties of a magnesium transporter of the SLC11/NRAMP family.
Ramanadane, K., Straub, M.S., Dutzler, R., Manatschal, C.(2022) Elife 11
- PubMed: 35001872
- DOI: https://doi.org/10.7554/eLife.74589
- Primary Citation of Related Structures:
7QIA, 7QIC, 7QJI, 7QJJ - PubMed Abstract:
Members of the ubiquitous SLC11/NRAMP family catalyze the uptake of divalent transition metal ions into cells. They have evolved to efficiently select these trace elements from a large pool of Ca 2+ and Mg 2+ , which are both orders of magnitude more abundant, and to concentrate them in the cytoplasm aided by the cotransport of H + serving as energy source. In the present study, we have characterized a member of a distant clade of the family found in prokaryotes, termed NRMTs, that were proposed to function as transporters of Mg 2+ . The protein transports Mg 2+ and Mn 2+ but not Ca 2+ by a mechanism that is not coupled to H + . Structures determined by cryo-EM and X-ray crystallography revealed a generally similar protein architecture compared to classical NRAMPs, with a restructured ion binding site whose increased volume provides suitable interactions with ions that likely have retained much of their hydration shell.
Organizational Affiliation:
Department of Biochemistry, University of Zurich, Zurich, Switzerland.