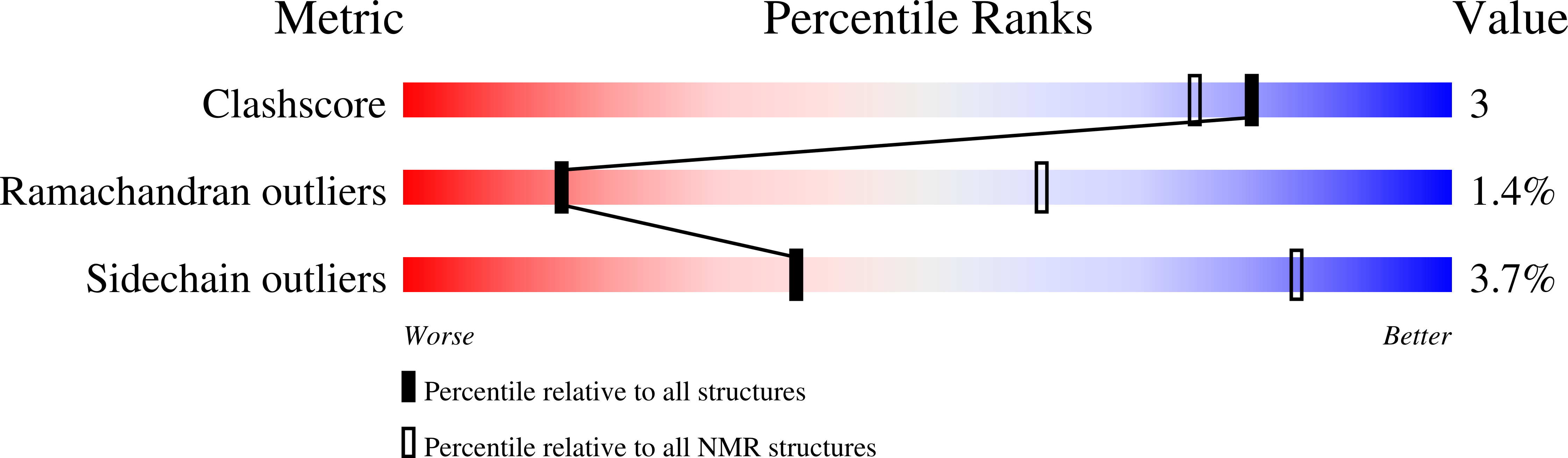
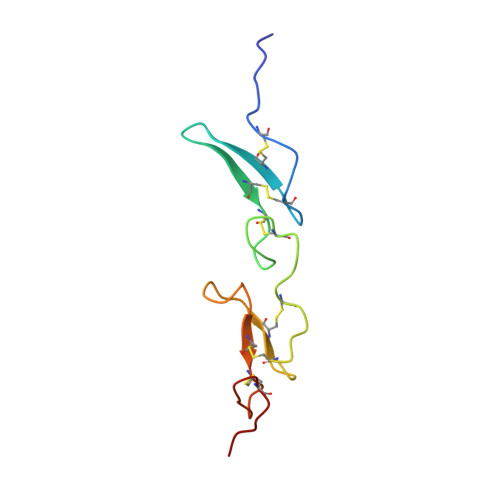
Solution structure of recombinant Pvfp-5 beta reveals insights into mussel adhesion.
Morando, M.A., Venturella, F., Sollazzo, M., Monaca, E., Sabbatella, R., Vetri, V., Passantino, R., Pastore, A., Alfano, C.(2022) Commun Biol 5: 739-739
- PubMed: 35879391
- DOI: https://doi.org/10.1038/s42003-022-03699-w
- Primary Citation of Related Structures:
7QAB - PubMed Abstract:
Some marine organisms can resist to aqueous tidal environments and adhere tightly on wet surface. This behavior has raised increasing attention for potential applications in medicine, biomaterials, and tissue engineering. In mussels, adhesive forces to the rock are the resultant of proteinic fibrous formations called byssus. We present the solution structure of Pvfp-5β, one of the three byssal plaque proteins secreted by the Asian green mussel Perna viridis, and the component responsible for initiating interactions with the substrate. We demonstrate that Pvfp-5β has a stably folded structure in agreement with the presence in the sequence of two EGF motifs. The structure is highly rigid except for a few residues affected by slow local motions in the µs-ms time scale, and differs from the model calculated by artificial intelligence methods for the relative orientation of the EGF modules, which is something where computational methods still underperform. We also show that Pvfp-5β is able to coacervate even with no DOPA modification, giving thus insights both for understanding the adhesion mechanism of adhesive mussel proteins, and developing of biomaterials.
Organizational Affiliation:
Structural Biology and Biophysics Unit, Fondazione Ri.MED, 90133, Palermo, Italy.