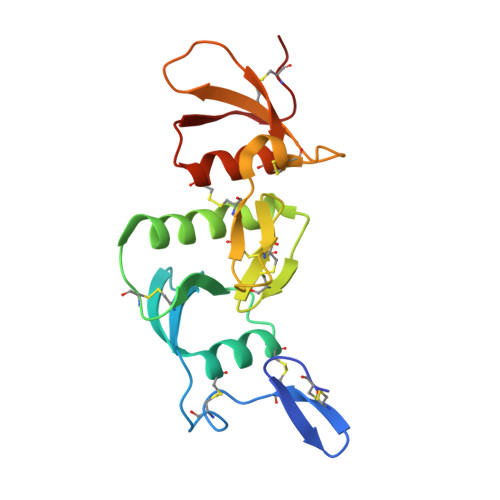
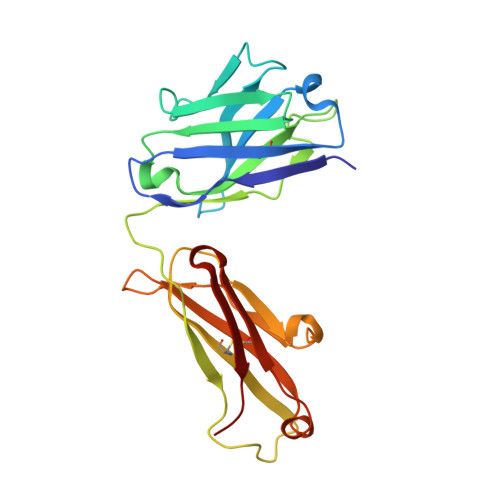
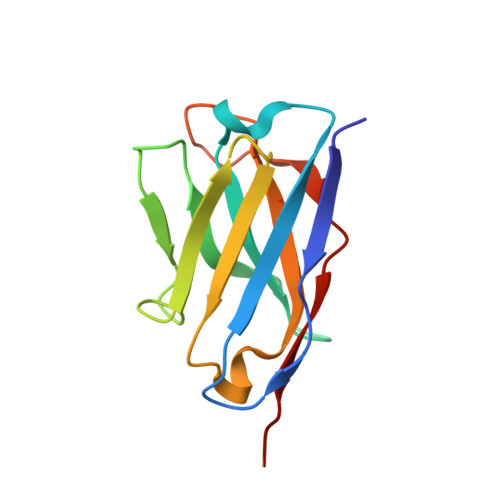
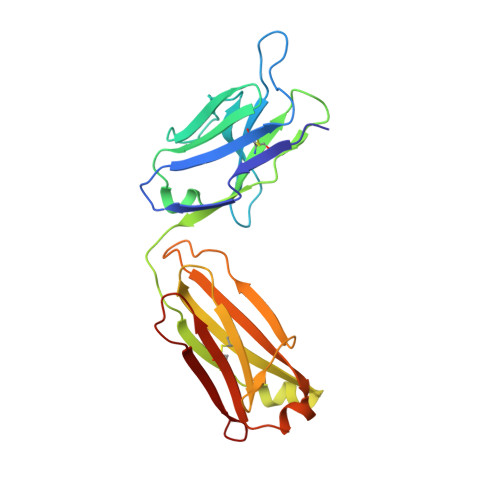
Development, Characterization, and in vivo Validation of a Humanized C6 Monoclonal Antibody that Inhibits the Membrane Attack Complex.
Gytz Olesen, H., Michailidou, I., Zelek, W.M., Vreijling, J., Ruizendaal, P., de Klein, F., Marquart, J.A., Kuipers, T.B., Mei, H., Zhang, Y., Ahasan, M., Johnson, K.K., Wang, Y., Morgan, B.P., van Dijk, M., Fluiter, K., Andersen, G.R., Baas, F.(2022) J Innate Immun : 1-21
- PubMed: 35551129
- DOI: https://doi.org/10.1159/000524587
- Primary Citation of Related Structures:
7Q6C - PubMed Abstract:
Damage and disease of nerves activates the complement system. We demonstrated that activation of the terminal pathway of the complement system leads to the formation of the membrane attack complex (MAC) and delays regeneration in the peripheral nervous system. Animals deficient in the complement component C6 showed improved recovery after neuronal trauma. Thus, inhibitors of the MAC might be of therapeutic use in neurological disease. Here, we describe the development, structure, mode of action, and properties of a novel therapeutic monoclonal antibody, CP010, against C6 that prevents formation of the MAC in vivo. The monoclonal antibody is humanized and specific for C6 and binds to an epitope in the FIM1-2 domain of human and primate C6 with sub-nanomolar affinity. Using biophysical and structural studies, we show that the anti-C6 antibody prevents the interaction between C6 and C5/C5b by blocking the C6 FIM1-2:C5 C345c axis. Systemic administration of the anti-C6 mAb caused complete depletion of free C6 in circulation in transgenic rats expressing human C6 and thereby inhibited MAC formation. The antibody prevented disease in experimental autoimmune myasthenia gravis and ameliorated relapse in chronic relapsing experimental autoimmune encephalomyelitis in human C6 transgenic rats. CP010 is a promising complement C6 inhibitor that prevents MAC formation. Systemic administration of this C6 monoclonal antibody has therapeutic potential in the treatment of neuronal disease.
Organizational Affiliation:
Department of Molecular Biology and Genetics - Protein Science, Aarhus University, Aarhus, Denmark.