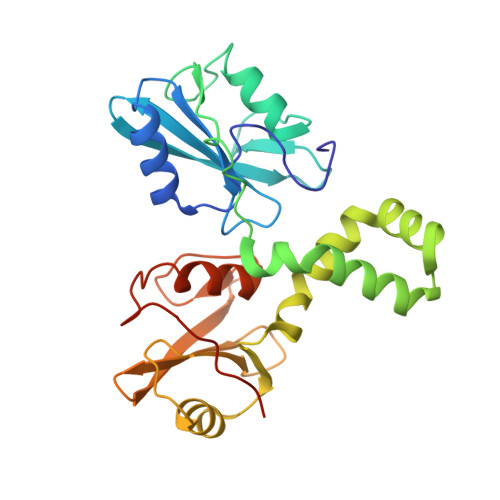
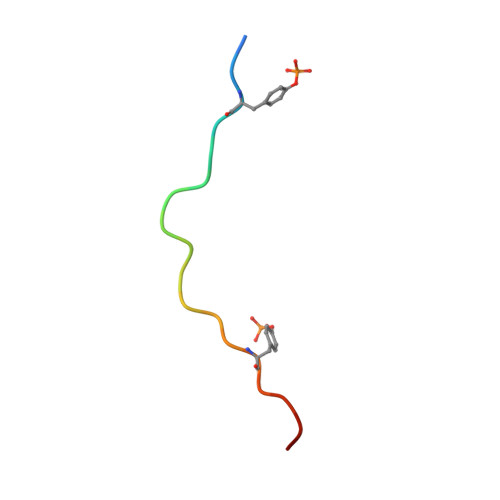
The mechanism of allosteric activation of SYK kinase derived from multiple phospho-ITAM-bound structures.
Bradshaw, W.J., Harris, G., Gileadi, O., Katis, V.L.(2024) Structure 32: 2337-2351.e4
- PubMed: 39442513
- DOI: https://doi.org/10.1016/j.str.2024.09.024
- Primary Citation of Related Structures:
7Q5T, 7Q5U, 7Q5W, 7Q63 - PubMed Abstract:
Spleen tyrosine kinase (SYK) is central to adaptive and innate immune signaling. It features a regulatory region containing tandem SH2 (tSH2) domains separated by a helical "hinge" segment keeping SYK inactive by associating with the kinase domain. SYK activation is triggered when the tSH2 domains bind to a phosphorylated immunoreceptor tyrosine-based activation motif (ITAM) found on receptor tails. Past mutational studies have indicated that ITAM binding disrupts the hinge-kinase interaction, leading to SYK phosphorylation and activation. However, the mechanism of this process is unclear, as the ITAM interaction occurs far from the hinge region. We have determined crystal structures of three phospho-ITAMs in complex with the tSH2 domains, revealing a highly conserved binding mechanism. These structures, together with mutational studies and biophysical analyses, reveal that phospho-ITAM binding restricts SH2 domain movement and causes allosteric changes in the hinge region. These changes are not compatible with the association of the kinase domain, leading to kinase activation.
Organizational Affiliation:
Alzheimer's Research UK Oxford Drug Discovery Institute, Centre for Medicines Discovery, Nuffield Department of Medicine Research Building, Old Road Campus, University of Oxford, Oxford OX3 7FZ, UK.