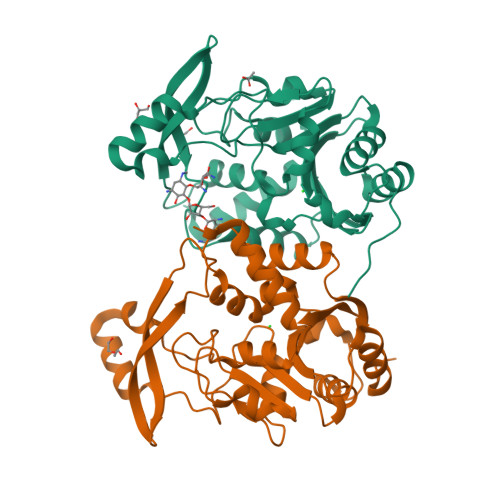
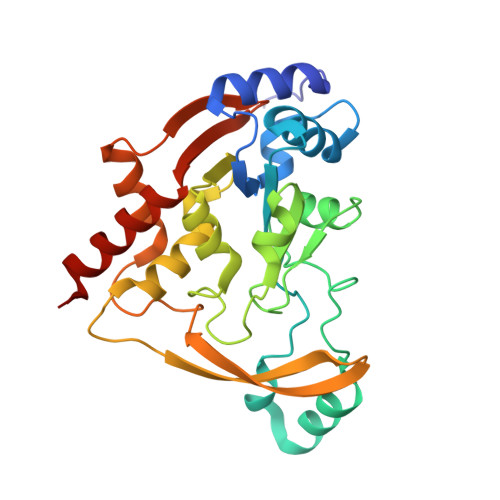
The 3-N-alkylation of the neomycin B outmaneuvers the aminoglycoside resistant enzyme acetyltransferase(3)IIIa via an unexpected mechanism
Pontillo, N., Warszawik, E.M., Partipilo, M., Stetsenko, A., Loznik, M., Yang, X., Slotboom, D.J., Guskov, A., Herrmann, A.To be published.