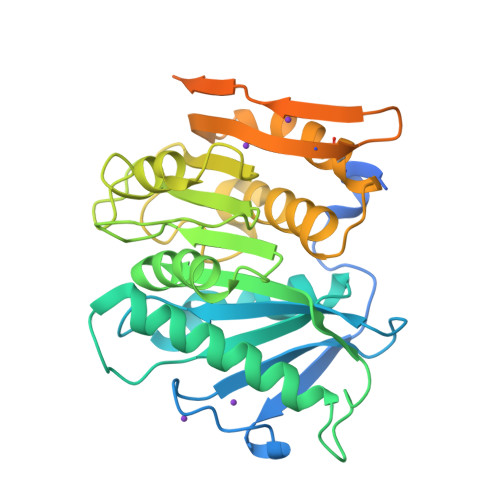
The Bacteroidetes Aequorivita sp. and Kaistella jeonii Produce Promiscuous Esterases With PET-Hydrolyzing Activity.
Zhang, H., Perez-Garcia, P., Dierkes, R.F., Applegate, V., Schumacher, J., Chibani, C.M., Sternagel, S., Preuss, L., Weigert, S., Schmeisser, C., Danso, D., Pleiss, J., Almeida, A., Hocker, B., Hallam, S.J., Schmitz, R.A., Smits, S.H.J., Chow, J., Streit, W.R.(2021) Front Microbiol 12: 803896-803896
- PubMed: 35069509
- DOI: https://doi.org/10.3389/fmicb.2021.803896
- Primary Citation of Related Structures:
7PZJ - PubMed Abstract:
Certain members of the Actinobacteria and Proteobacteria are known to degrade polyethylene terephthalate (PET). Here, we describe the first functional PET-active enzymes from the Bacteroidetes phylum. Using a PETase-specific Hidden-Markov-Model- (HMM-) based search algorithm, we identified several PETase candidates from Flavobacteriaceae and Porphyromonadaceae. Among them, two promiscuous and cold-active esterases derived from Aequorivita sp. (PET27) and Kaistella jeonii (PET30) showed depolymerizing activity on polycaprolactone (PCL), amorphous PET foil and on the polyester polyurethane Impranil ® DLN. PET27 is a 37.8 kDa enzyme that released an average of 174.4 nmol terephthalic acid (TPA) after 120 h at 30°C from a 7 mg PET foil platelet in a 200 μl reaction volume, 38-times more than PET30 (37.4 kDa) released under the same conditions. The crystal structure of PET30 without its C-terminal Por-domain (PET30ΔPorC) was solved at 2.1 Å and displays high structural similarity to the Is PETase. PET30 shows a Phe-Met-Tyr substrate binding motif, which seems to be a unique feature, as Is PETase, LCC and PET2 all contain Tyr-Met-Trp binding residues, while PET27 possesses a Phe-Met-Trp motif that is identical to Cut190. Microscopic analyses showed that K. jeonii cells are indeed able to bind on and colonize PET surfaces after a few days of incubation. Homologs of PET27 and PET30 were detected in metagenomes, predominantly aquatic habitats, encompassing a wide range of different global climate zones and suggesting a hitherto unknown influence of this bacterial phylum on man-made polymer degradation.
Organizational Affiliation:
Department of Microbiology and Biotechnology, University of Hamburg, Hamburg, Germany.