Structural and functional investigation of the human snRNP assembly factor AAR2 in complex with the RNase H-like domain of PRPF8.
Preussner, M., Santos, K.F., Alles, J., Heroven, C., Heyd, F., Wahl, M.C., Weber, G.(2022) Acta Crystallogr D Struct Biol 78: 1373-1383
- PubMed: 36322420
- DOI: https://doi.org/10.1107/S2059798322009755
- Primary Citation of Related Structures:
7PJH - PubMed Abstract:
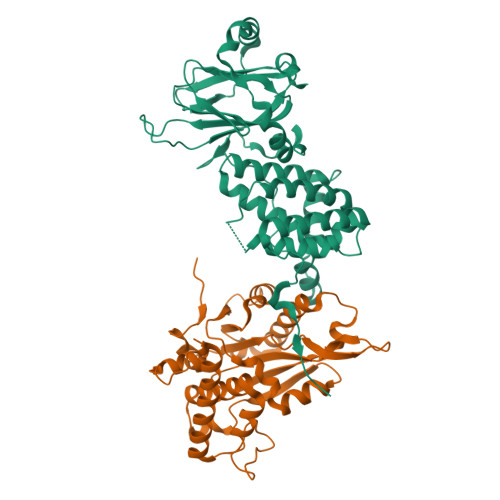
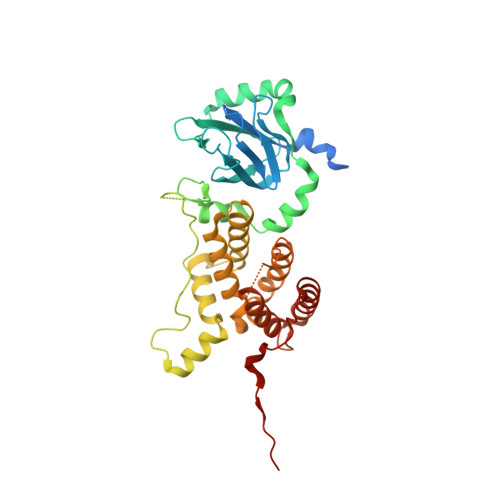
Small nuclear ribonucleoprotein complexes (snRNPs) represent the main subunits of the spliceosome. While the assembly of the snRNP core particles has been well characterized, comparably little is known of the incorporation of snRNP-specific proteins and the mechanisms of snRNP recycling. U5 snRNP assembly in yeast requires binding of the the Aar2 protein to Prp8p as a placeholder to preclude premature assembly of the SNRNP200 helicase, but the role of the human AAR2 homolog has not yet been investigated in detail. Here, a crystal structure of human AAR2 in complex with the RNase H-like domain of the U5-specific PRPF8 (PRP8F RH) is reported, revealing a significantly different interaction between the two proteins compared with that in yeast. Based on the structure of the AAR2-PRPF8 RH complex, the importance of the interacting regions and residues was probed and AAR2 variants were designed that failed to stably bind PRPF8 in vitro. Protein-interaction studies of AAR2 with U5 proteins using size-exclusion chromatography reveal similarities and marked differences in the interaction patterns compared with yeast Aar2p and imply phosphorylation-dependent regulation of AAR2 reminiscent of that in yeast. It is found that in vitro AAR2 seems to lock PRPF8 RH in a conformation that is only compatible with the first transesterification step of the splicing reaction and blocks a conformational switch to the step 2-like, Mg 2+ -coordinated conformation that is likely during U5 snRNP biogenesis. These findings extend the picture of AAR2 PRP8 interaction from yeast to humans and indicate a function for AAR2 in the spliceosomal assembly process beyond its role as an SNRNP200 placeholder in yeast.
Organizational Affiliation:
Laboratory of RNA Biochemistry, Freie Universität Berlin, Takustrasse 6, 14195 Berlin, Germany.