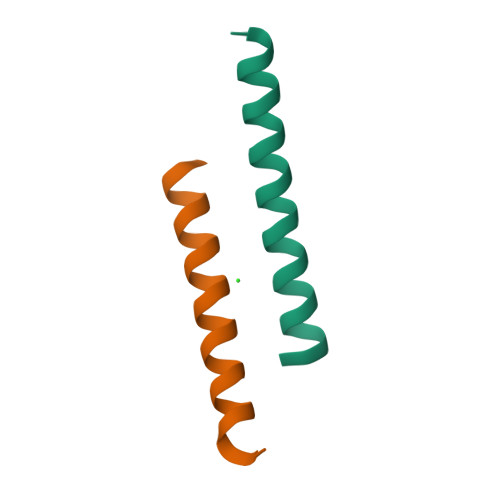
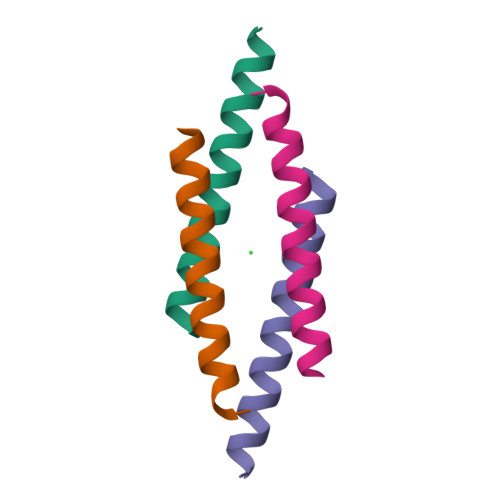
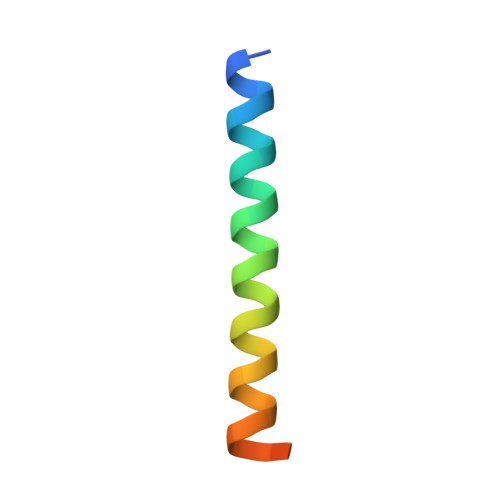
The structure of the antimicrobial human cathelicidin LL-37 shows oligomerization and channel formation in the presence of membrane mimics.
Sancho-Vaello, E., Gil-Carton, D., Francois, P., Bonetti, E.J., Kreir, M., Pothula, K.R., Kleinekathofer, U., Zeth, K.(2020) Sci Rep 10: 17356-17356
- PubMed: 33060695
- DOI: https://doi.org/10.1038/s41598-020-74401-5
- Primary Citation of Related Structures:
7PDC - PubMed Abstract:
The human cathelicidin LL-37 serves a critical role in the innate immune system defending bacterial infections. LL-37 can interact with molecules of the cell wall and perforate cytoplasmic membranes resulting in bacterial cell death. To test the interactions of LL-37 and bacterial cell wall components we crystallized LL-37 in the presence of detergents and obtained the structure of a narrow tetrameric channel with a strongly charged core. The formation of a tetramer was further studied by cross-linking in the presence of detergents and lipids. Using planar lipid membranes a small but defined conductivity of this channel could be demonstrated. Molecular dynamic simulations underline the stability of this channel in membranes and demonstrate pathways for the passage of water molecules. Time lapse studies of E. coli cells treated with LL-37 show membrane discontinuities in the outer membrane followed by cell wall damage and cell death. Collectively, our results open a venue to the understanding of a novel AMP killing mechanism and allows the rational design of LL-37 derivatives with enhanced bactericidal activity.
Organizational Affiliation:
Unidad de Biofisica, Centro Mixto Consejo Superior de Investigaciones Cientificas-Universidad del País Vasco/Euskal Herriko Unibertsitatea (CSIC, UPV/EHU), Barrio Sarriena s/n, Leioa, Bizkaia, Spain.