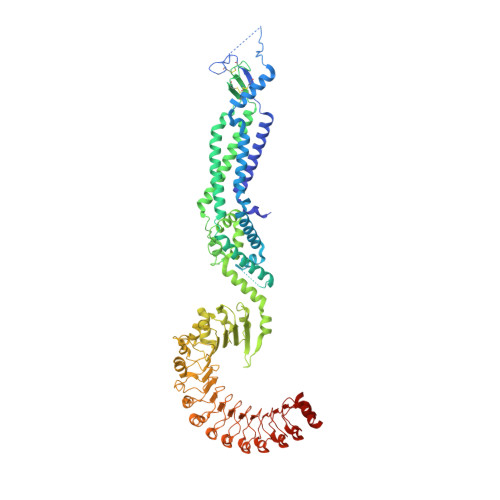
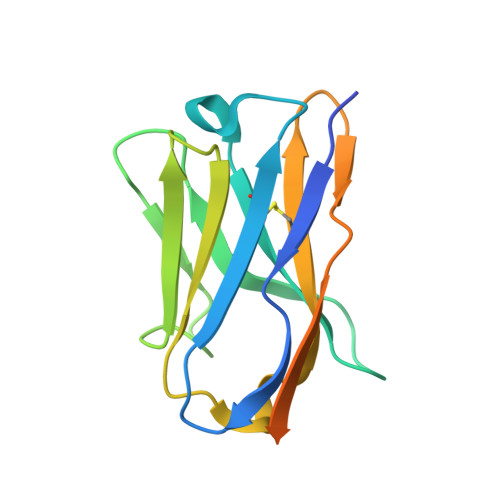
Allosteric modulation of LRRC8 channels by targeting their cytoplasmic domains.
Deneka, D., Rutz, S., Hutter, C.A.J., Seeger, M.A., Sawicka, M., Dutzler, R.(2021) Nat Commun 12: 5435-5435
- PubMed: 34521847
- DOI: https://doi.org/10.1038/s41467-021-25742-w
- Primary Citation of Related Structures:
7P5V, 7P5W, 7P5Y, 7P60, 7P6K - PubMed Abstract:
Members of the LRRC8 family form heteromeric assemblies, which function as volume-regulated anion channels. These modular proteins consist of a transmembrane pore and cytoplasmic leucine-rich repeat (LRR) domains. Despite their known molecular architecture, the mechanism of activation and the role of the LRR domains in this process has remained elusive. Here we address this question by generating synthetic nanobodies, termed sybodies, which target the LRR domain of the obligatory subunit LRRC8A. We use these binders to investigate their interaction with homomeric LRRC8A channels by cryo-electron microscopy and the consequent effect on channel activation by electrophysiology. The five identified sybodies either inhibit or enhance activity by binding to distinct epitopes of the LRR domain, thereby altering channel conformations. In combination, our work provides a set of specific modulators of LRRC8 proteins and reveals the role of their cytoplasmic domains as regulators of channel activity by allosteric mechanisms.
Organizational Affiliation:
Department of Biochemistry University of Zurich, Winterthurerstrasse 190, CH-8057, Zurich, Switzerland.