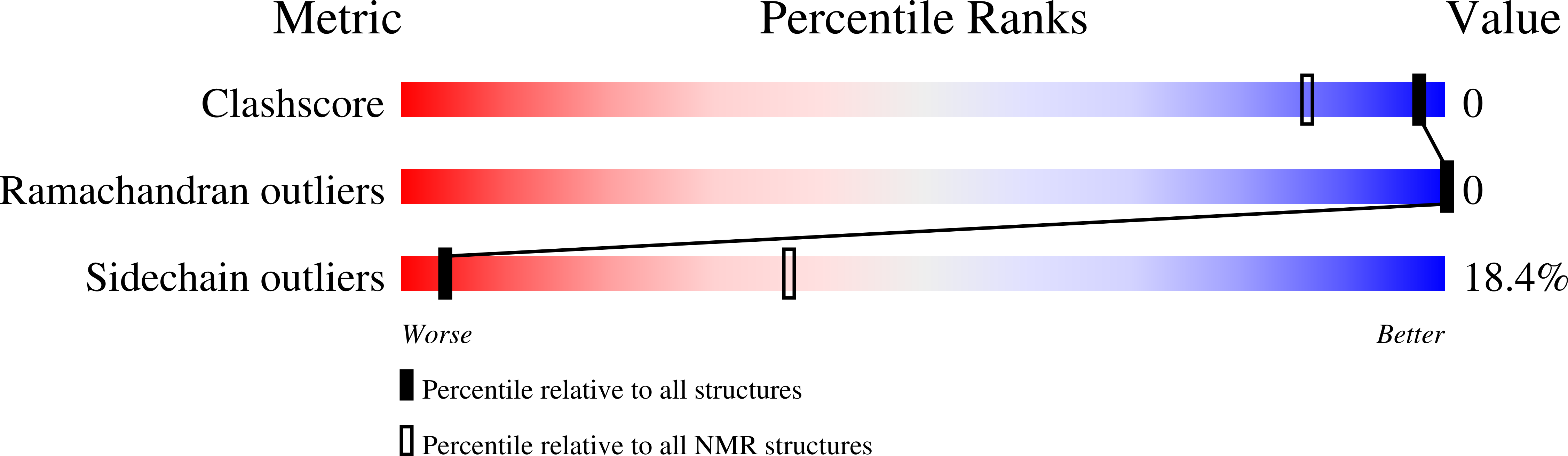Structural analysis of a simplified model reproducing SARS-CoV-2 S RBD/ACE2 binding site.
Buonocore, M., Santoro, A., Grimaldi, M., Covelli, V., Firoznezhad, M., Rodriquez, M., Santin, M., D'Ursi, A.M.(2022) Heliyon 8: e11568-e11568
- PubMed: 36406731
- DOI: https://doi.org/10.1016/j.heliyon.2022.e11568
- Primary Citation of Related Structures:
7P55, 7P5G, 7P5Q, 7P5S - PubMed Abstract:
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is an RNA virus identified as the cause of the coronavirus outbreak in December 2019 (COVID-19). Like all the RNA viruses, SARS-CoV-2 constantly evolves through mutations in its genome, accumulating 1-2 nucleotide changes every month, giving the virus a selective advantage through enhanced transmissibility, greater pathogenicity, and the possibility of circumventing immunity previously acquired by an individual either by natural infection or by vaccination. Several SARS-CoV-2 variants of concern (VoC) have been identified, among which we find Alpha (Lineage B.1.1.7), Beta (Lineage B.1.351), and Gamma (Lineage P.1) variants. Most of the mutations occur in the spike (S) protein, a surface glycoprotein that plays a crucial role in viral infection; the S protein binds the host cell receptor, the angiotensin-converting enzyme of type 2 (ACE2) via the receptor binding domain (RBD) and catalyzes the fusion of the viral membrane with the host cell. In this work, we present the development of a simplified system that would afford to study the change in the SARS-CoV-2 S RBD/ACE2 binding related to the frequent mutations. In particular, we synthesized and studied the structure of short amino acid sequences, mimicking the two proteins' critical portions. Variations in the residues were easily managed through the one-point alteration of the sequences. Nuclear magnetic resonance (NMR) and circular dichroism (CD) spectroscopies provide insights into ACE2 and SARS-CoV-2 S RBD structure with its related three variants (Alpha, Beta, and Gamma). Spectroscopy data supported by molecular dynamics lead to the description of an ACE2/RBD binding model in which the effect of a single amino acid mutation in changing the binding of S protein to the ACE2 receptor is predictable.
Organizational Affiliation:
University of Salerno, Department of Pharmacy, Via Giovanni Paolo II, 132-84084 Fisciano, Salerno, Italy.