Toward the Design of Ligands Selective for the C-Terminal Domain of TEADs.
Liberelle, M., Toulotte, F., Renault, N., Gelin, M., Allemand, F., Melnyk, P., Guichou, J.F., Cotelle, P.(2022) J Med Chem 65: 5926-5940
- PubMed: 35389210
- DOI: https://doi.org/10.1021/acs.jmedchem.2c00075
- Primary Citation of Related Structures:
7OYJ - PubMed Abstract:
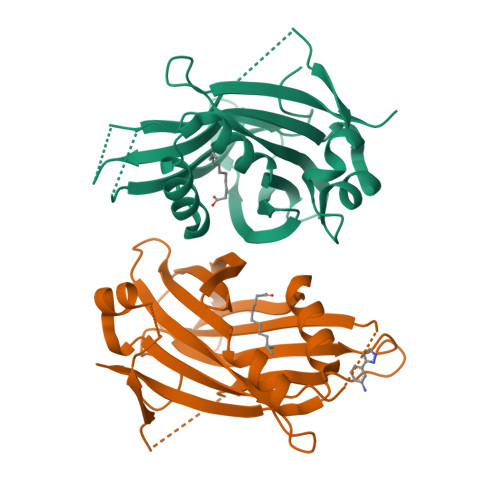
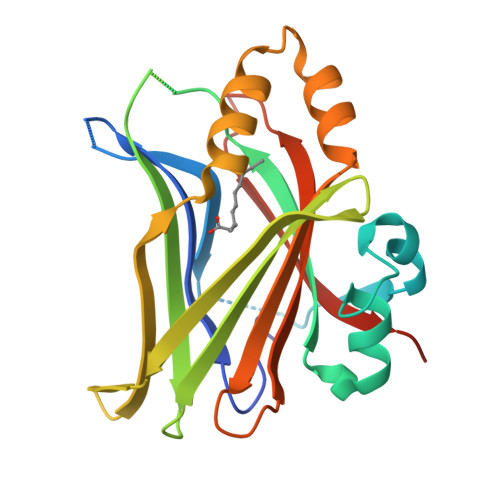
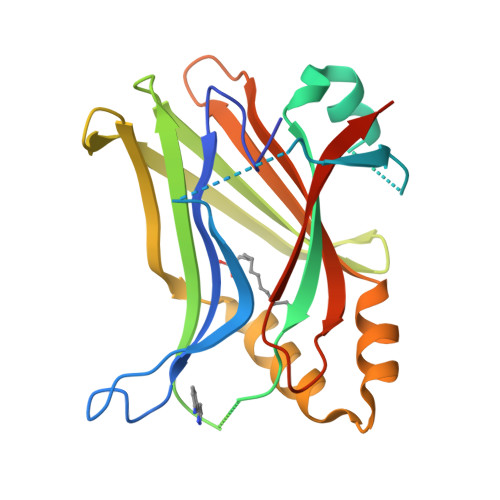
The Hippo signaling pathway plays a fundamental role in the control of organ growth, cell proliferation, and stem cell characters. TEADs are the main transcriptional output regulators of the Hippo signaling pathway and bind to YAP and TAZ co-activators. TEAD1-4 are expressed differently, depending on the tissue and developmental level, and can be overexpressed in certain pathologies. TEAD ligands mainly target the internal pocket of the C-terminal domain of TEAD, and the first ligands selective for TEAD1 and TEAD3 have been recently reported. In this paper, we focus on the topographic homology of the TEAD C-terminal domain both externally and in the internal pocket to highlight the possibility of rationally designing ligands selective for one of the TEAD family members. We identified a novel TEAD2-specific pocket and reported its first ligand. Finally, AlphaFold2 models of full-length TEADs suggest TEAD autoregulation and emphasize the importance of the interface 2.
Organizational Affiliation:
INSERM, CHU Lille, UMR-S 1172, Lille Neuroscience and Cognition Research Center, Université de Lille, F-59000 Lille, France.