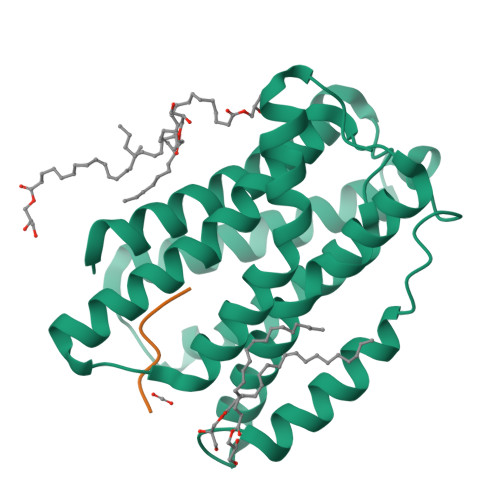
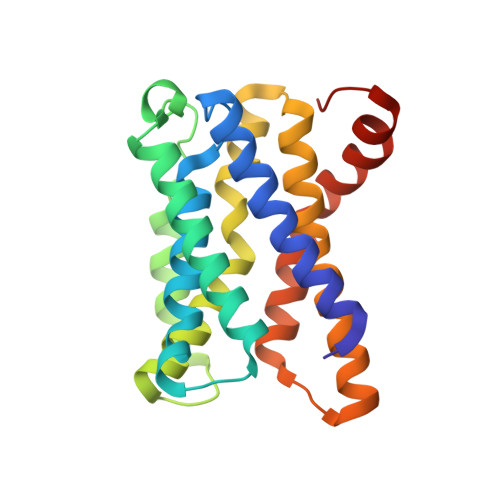
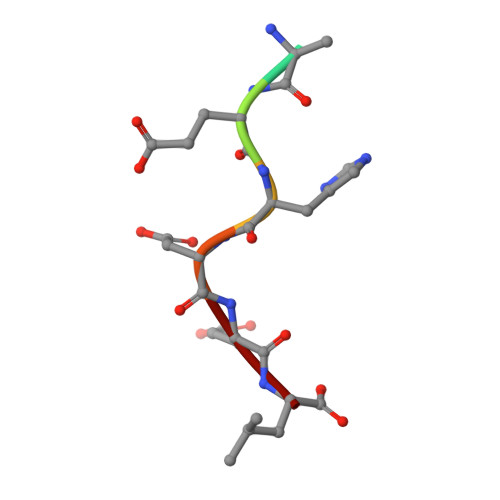
Molecular basis for pH sensing in the KDEL trafficking receptor.
Wu, Z., Smith, K., Gerondopoulos, A., Sobajima, T., Parker, J.L., Barr, F.A., Newstead, S., Biggin, P.C.(2024) Structure 32: 866-877.e4
- PubMed: 38626766
- DOI: https://doi.org/10.1016/j.str.2024.03.013
- Primary Citation of Related Structures:
7OXE, 7OYE, 8APY - PubMed Abstract:
Trafficking receptors control protein localization through the recognition of specific signal sequences that specify unique cellular locations. Differences in luminal pH are important for the vectorial trafficking of cargo receptors. The KDEL receptor is responsible for maintaining the integrity of the ER by retrieving luminally localized folding chaperones in a pH-dependent mechanism. Structural studies have revealed the end states of KDEL receptor activation and the mechanism of selective cargo binding. However, precisely how the KDEL receptor responds to changes in luminal pH remains unclear. To explain the mechanism of pH sensing, we combine analysis of X-ray crystal structures of the KDEL receptor at neutral and acidic pH with advanced computational methods and cell-based assays. We show a critical role for ordered water molecules that allows us to infer a direct connection between protonation in different cellular compartments and the consequent changes in the affinity of the receptor for cargo.
Organizational Affiliation:
Structural Bioinformatics and Computational Biochemistry, Department of Biochemistry, University of Oxford, Oxford OX1 3QU, UK.