Discovery of Novel Inhibitors of Uridine Diphosphate- N -Acetylenolpyruvylglucosamine Reductase (MurB) from Pseudomonas aeruginosa , an Opportunistic Infectious Agent Causing Death in Cystic Fibrosis Patients.
Acebron-Garcia-de-Eulate, M., Mayol-Llinas, J., Holland, M.T.O., Kim, S.Y., Brown, K.P., Marchetti, C., Hess, J., Di Pietro, O., Mendes, V., Abell, C., Floto, R.A., Coyne, A.G., Blundell, T.L.(2022) J Med Chem 65: 2149-2173
- PubMed: 35080396
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01684
- Primary Citation of Related Structures:
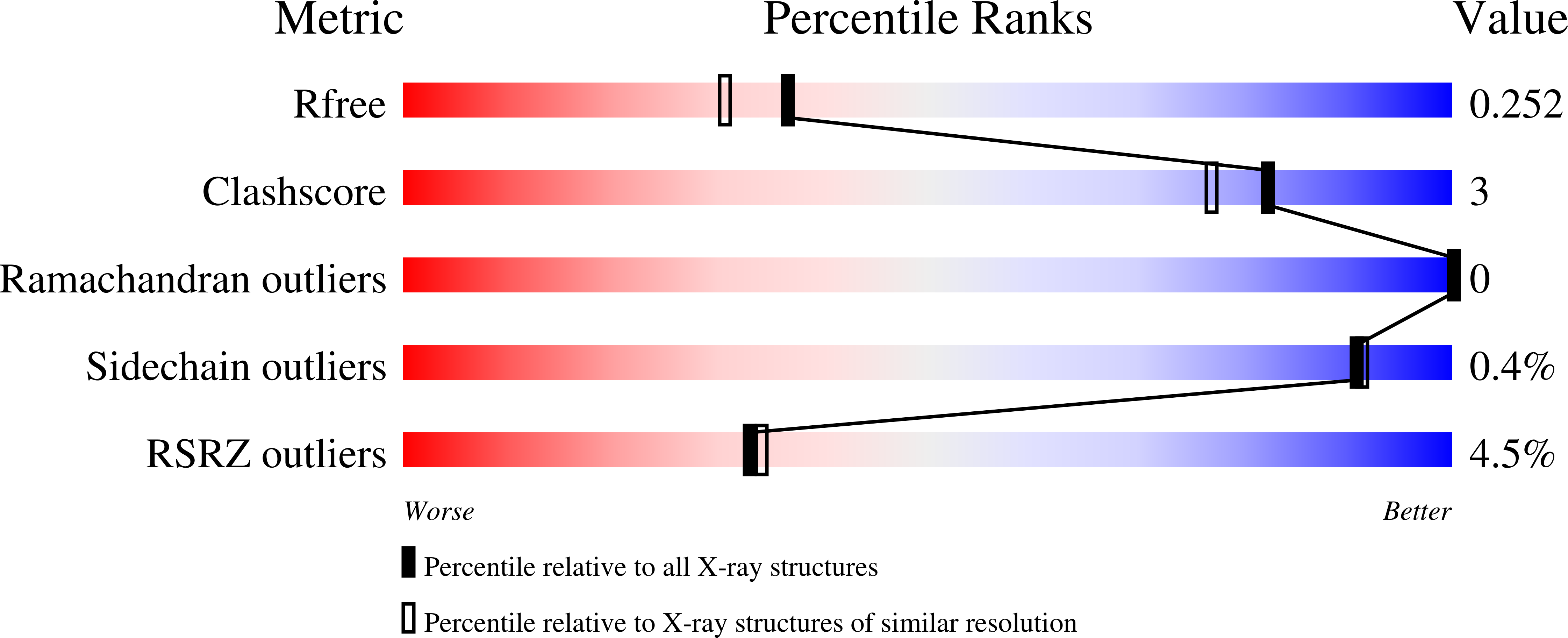
7OR2, 7ORZ, 7OSQ - PubMed Abstract:
Pseudomonas aeruginosa is of major concern for cystic fibrosis patients where this infection can be fatal. With the emergence of drug-resistant strains, there is an urgent need to develop novel antibiotics against P. aeruginosa . MurB is a promising target for novel antibiotic development as it is involved in the cell wall biosynthesis. MurB has been shown to be essential in P. aeruginosa , and importantly, no MurB homologue exists in eukaryotic cells. A fragment-based drug discovery approach was used to target Pa MurB. This led to the identification of a number of fragments, which were shown to bind to MurB. One fragment, a phenylpyrazole scaffold, was shown by ITC to bind with an affinity of K d = 2.88 mM (LE 0.23). Using a structure guided approach, different substitutions were synthesized and the initial fragment was optimized to obtain a small molecule with K d = 3.57 μM (LE 0.35).
Organizational Affiliation:
Department of Biochemistry, University of Cambridge, Tennis Court Road, Cambridge CB2 1GA, U.K.