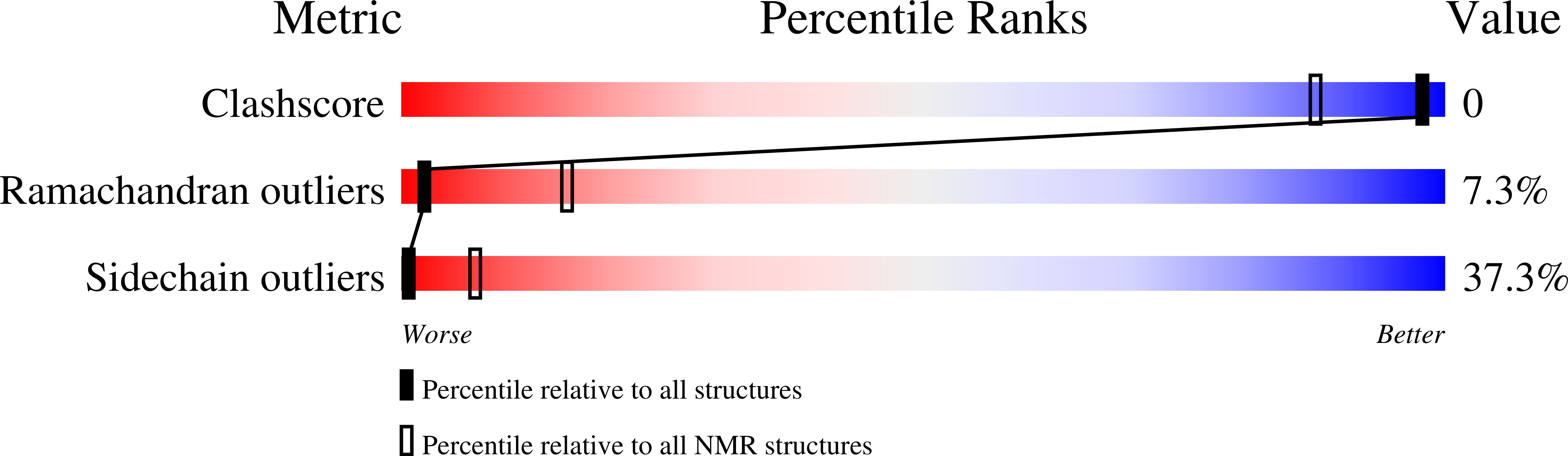First-in-Class Cyclic Temporin L Analogue: Design, Synthesis, and Antimicrobial Assessment.
Bellavita, R., Casciaro, B., Di Maro, S., Brancaccio, D., Carotenuto, A., Falanga, A., Cappiello, F., Buommino, E., Galdiero, S., Novellino, E., Grossmann, T.N., Mangoni, M.L., Merlino, F., Grieco, P.(2021) J Med Chem 64: 11675-11694
- PubMed: 34296619
- DOI: https://doi.org/10.1021/acs.jmedchem.1c01033
- Primary Citation of Related Structures:
7OS8, 7OSD - PubMed Abstract:
The pharmacodynamic and pharmacokinetic properties of bioactive peptides can be modulated by introducing conformational constraints such as intramolecular macrocyclizations, which can involve either the backbone and/or side chains. Herein, we aimed at increasing the α-helicity content of temporin L, an isoform of an intriguing class of linear antimicrobial peptides (AMPs), endowed with a wide antimicrobial spectrum, by the employment of diverse side-chain tethering strategies, including lactam, 1,4-substituted [1,2,3]-triazole, hydrocarbon, and disulfide linkers. Our approach resulted in a library of cyclic temporin L analogues that were biologically assessed for their antimicrobial, cytotoxic, and antibiofilm activities, leading to the development of the first-in-class cyclic peptide related to this AMP family. Our results allowed us to expand the knowledge regarding the relationship between the α-helical character of temporin derivatives and their biological activity, paving the way for the development of improved antibiotic cyclic AMP analogues.
Organizational Affiliation:
Department of Pharmacy, University of Naples "Federico II", Naples 80131, Italy.